MATURE iPSC-CARDIOMYOCYTES IN 48 HOURS
The use of human induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) is limited by functional immaturity, including immature calcium handling and contractility function. Physical conditioning of iPSC-CMs via electrical or mechanical stimulation facilitates maturation as measured by a positive force-frequency relationship and detection of positive inotropes. Current in vitro protocols require 2-4 weeks of chronic pacing. Using array-based contractility and local electrical stimulation, the Maestro elicits functionally mature phenotypes in iPSC-CMs after only 48 hours of chronic pacing.
What is High-Res contractility?
When cardiomyocytes are plated over a microelectrode array, they form a spontaneously beating syncytium. As the cells contract and relax, cells change shape and alter the coverage over the electrodes. This change can be measured as a change in impedance, or contractility. The Maestro offers high resolution contractility by sampling local contractility from many locations across the whole culture.
Detect changes in cardiomyocyte contractility
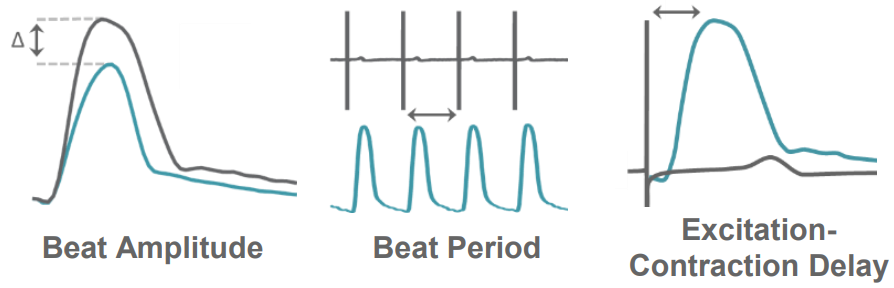
In the Maestro, contractility is measured from each electrode, providing local measurements of cardiomyocyte contractility in high spatial resolution across the culture. AxIS Navigator provides measures of both electrode and well-wide contractility, including Beat Amplitude, Beat Period, and Excitation-Contraction Delay. These measures can reveal changes in contractility as a result of maturation and compound treatment.
THE MAESTRO ADVANTAGE
- Track local changes in contractility
- Enable contractility with 3D constructs, such as spheroids and organoids
- Elicit mature phenotypes with only 48 hours of chronic pacing
- Measure cultures with wide variations in cell coverage and attachment
- Save incubator space with built-in environmental controls
FUNCTIONAL MATURITY IN 48 HOURS
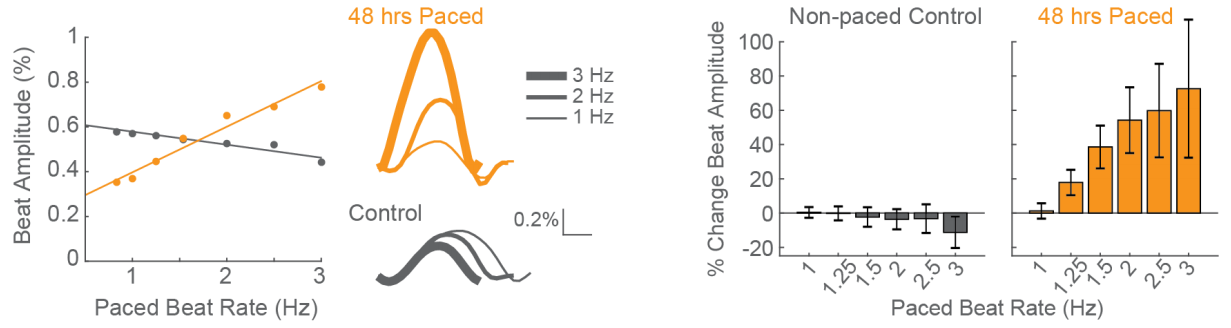
Positive force-frequency relationship after only 48 hours of chronic pacing
CDI iCell CM2 cardiomyocytes were chronically paced at 2 Hz for 48 hours using a CytoView MEA 24. After pacing, the cells were paced again at 0.8 to 3 Hz to assess the force-frequency relationship. Paced wells (orange) exhibited a positive force-frequency relationship after only 48 hours, while non-paced control wells (gray) showed a subtle negative relationship. Inset shows representative paced and non-paced control contractility traces at 1 Hz, 2 Hz, and 3 Hz.
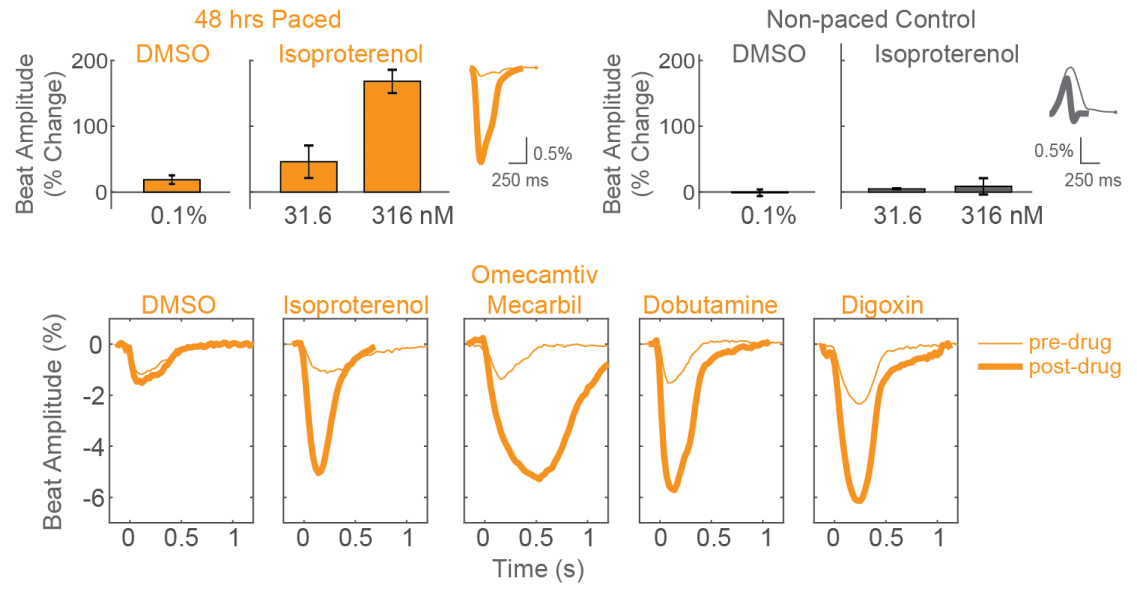
Detect positive inotropes after only 48 hours of chronic pacing
After chronic pacing at 2 Hz for 48 hours, CDI iCell CM2 cardiomyocytes were dosed with positive inotropes, such as isoproterenol. Chronically paced wells (orange) showed a dose-dependent increase in beat amplitude in response to isoproterenol, while non-paced control wells (gray) showed no response. Similarly, chronically paced wells also successfully detected a variety of other positive inotropes.