Immunotherapy is advancing rapidly, creating lifesaving treatments that use the body’s own immune system to target and destroy tumors. Transform your immuno-oncology research and simplify your workflow with our easy-to-use immune cell killing assays. Quickly and efficiently assess potential candidates and reliable, functional data for decision making. Discover how Axion’s live-cell analysis platforms can accelerate your immunotherapy research and development—from investigating immunomodulators to evaluating CAR cell killing in solid and liquid tumors.
Immuno-oncology assays: Get more with live-cell analysis
There are many critical steps in development. The right assay can help you make the right choices.
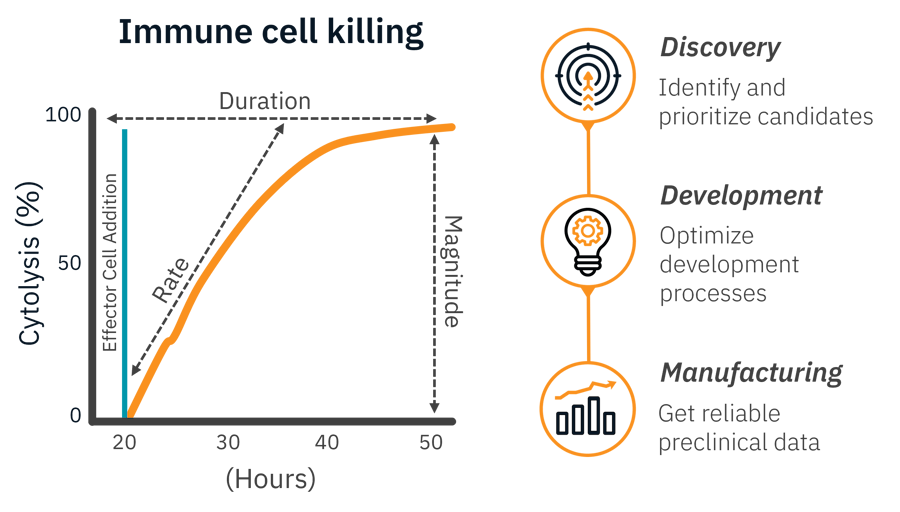
Our live-cell assays capture the entire experiment. Analyze rate, duration, and magnitude with a single, easy assay.
Identify and prioritize candidates
- Screen checkpoint inhibitors, compare CAR constructs, or test immunomodulators
Optimize your development process
- Evaluate transduction methods, proliferation, or cryopreservation with clear functional data
Get reliable preclinical data
- No critical reagents to validate, just clean reproducible potency data with GMP compatible systems available.
Explore more immuno-oncology assay:
CAR T Cell Killing Assay
T cells that recognize specific antigens on the tumor cell through genetically engineered receptors. Learn more >>
Natural Killer Cell Assay
NK cells are granular lymphocytes involved in the innate immune response to viruses and tumors. Learn more >>
Antibody-dependent Cellular Cytotoxicity (ADCC)
By binding to tumor cells, antibodies can enhance the recognition and killing by immune cells. Learn more >>
Solid Tumor/Cancer Spheroid
Immune penetration and the tumor microenvironment can challenge immunotherapies. Investigate immune cell killing in cancer spheroids. Learn more >>
Liquid Tumor Assay
Liquid tumors such as leukemia, lymphoma, and myeloma are an important target in immuno-oncology. Learn more >>

“... we use Axion BioSystems' Maestro Z system from early discovery to product manufacturing to demonstrate the functional cancer cell killing”
“For advanced cell therapies, it is more important than ever to optimize development and demonstrate the functional activity of the finished cell product before it is administered to the patient. At Immupeutics Medicine, we use Axion BioSystems' Maestro Z system from early discovery to product manufacturing to demonstrate the functional cancer cell killing potential of our IPM001 CTL (Multi Tumor-Antigens Cytotoxic T Lymphocyte Cell) cell therapy.”
Henghui Zhang, M.D., Ph.D, Co-Founder, Chairman and CEO of Immupeutics


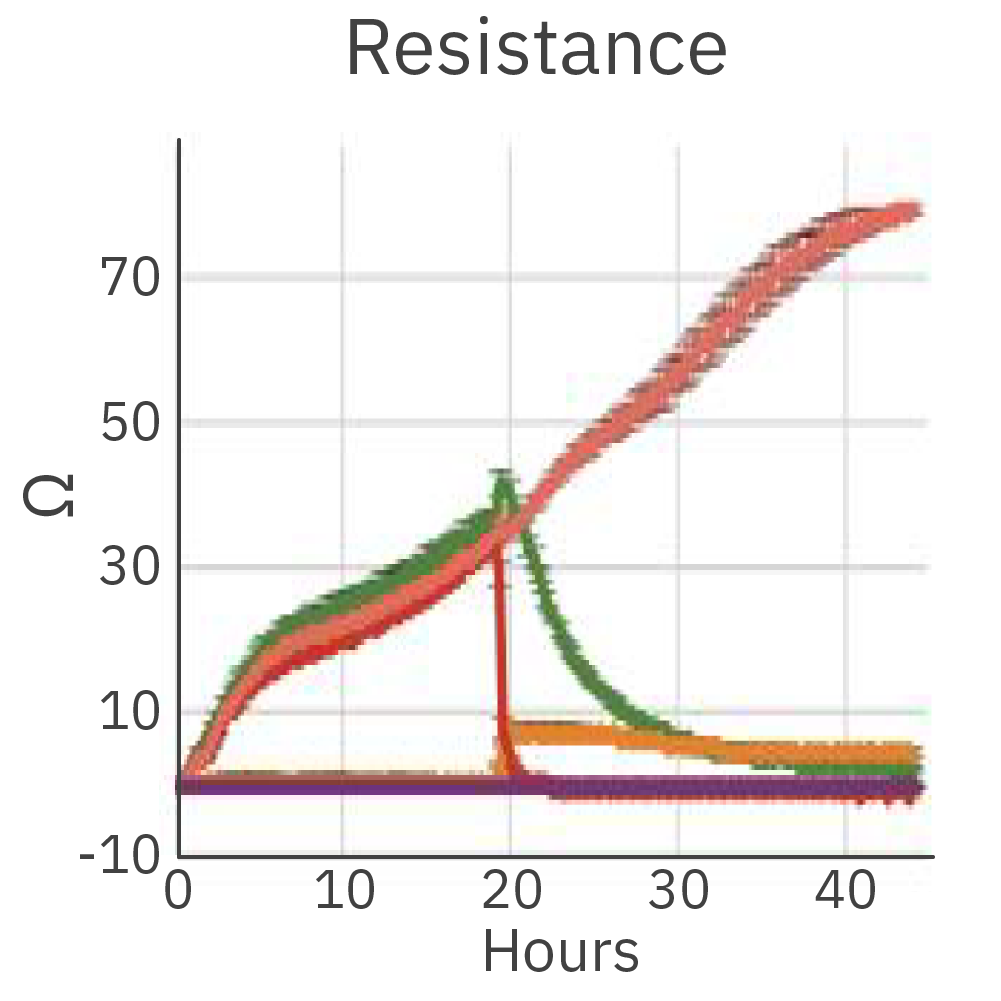
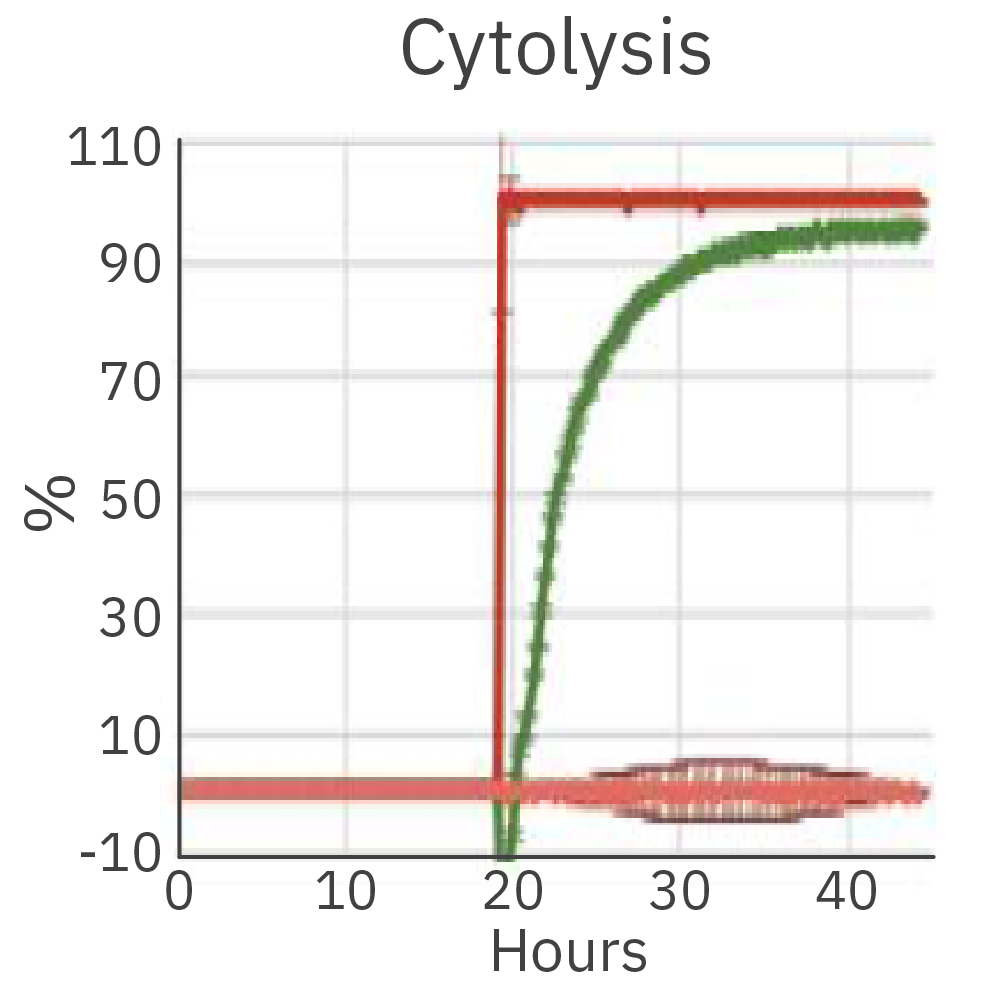
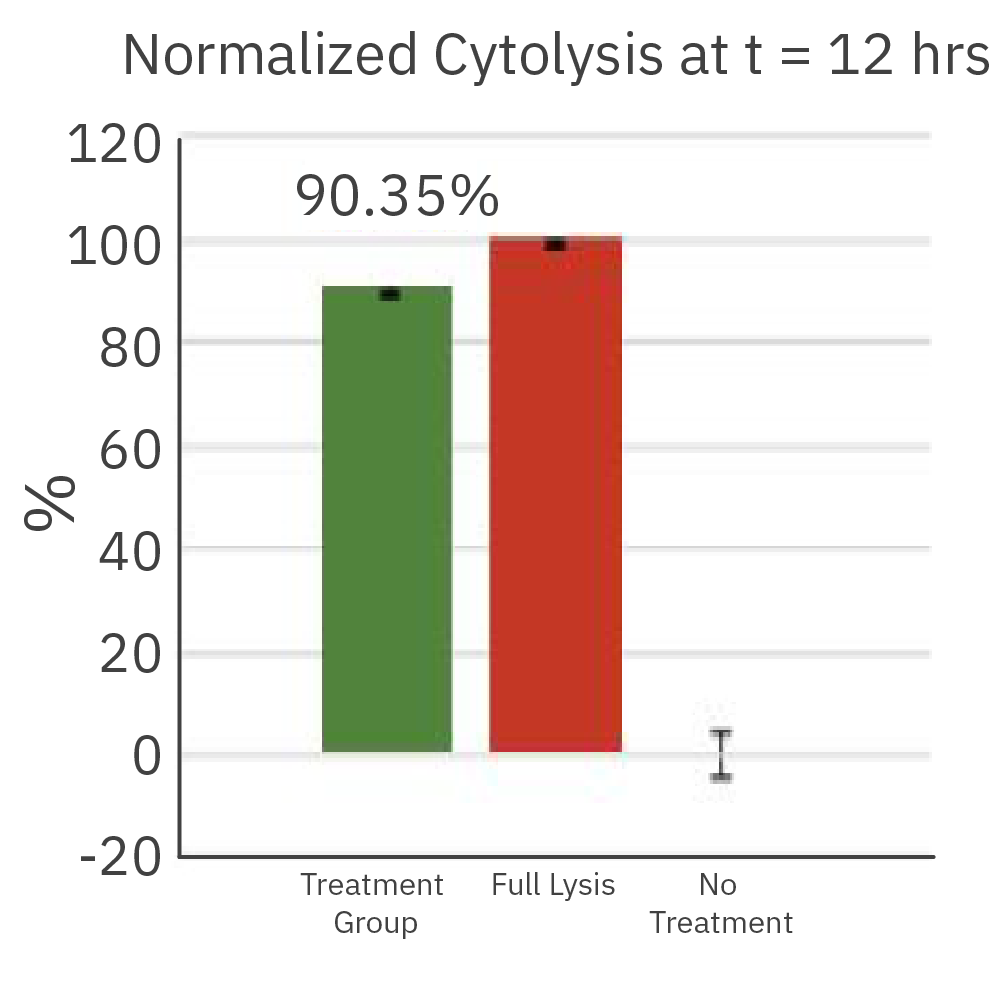
The cytolysis reached 90.35% after 12 hours of co-culture of the effector cells (CTL2) and the cancer target cells.