While neural cultures are often spontaneously active, electrical and optical stimulation offers enhanced control of neural activity. Electrical stimulation can be used to study network connectivity, evaluate excitability, and reduce variability between replicates. Optogenetics can be used to further customize an experiment with the ability to stimulate or suppress activity within specific subpopulations of a culture.
The Maestro MEA platform provides noninvasive electrical activity recordings across each neural network, while the Lumos optical stimulation system delivers precisely controlled light. Together, these devices seamlessly pair to unlock entirely new assay capabilities, in a high-throughput format.
-
Powering neurons with light: optogenetic control of two neural populations>
-
Evoked neural activity for seizurogenic screening>
-
Chronos enables high frequency responses without adaptation>
-
High-frequency stimulation to probe excitatory-inhibitory balance>
-
Assay Steps>
Using the Lumos optical stimulator and Axion's multiwell Maestro MEA system to study neural network activity with optogenetics. Neurons on the left side of the well have been transduced with a blue light-activated ion channel (channel rhodopsin, ChR2), the neurons on the right have not. Watch as blue light induces activity in the ChR2-labeled left-side neural population, that then spreads to, and activates the unlabeled right-side neural population. After the addition of a cocktail of synaptic blockers the neural cells on the left can no longer drive the cells on the right to fire.
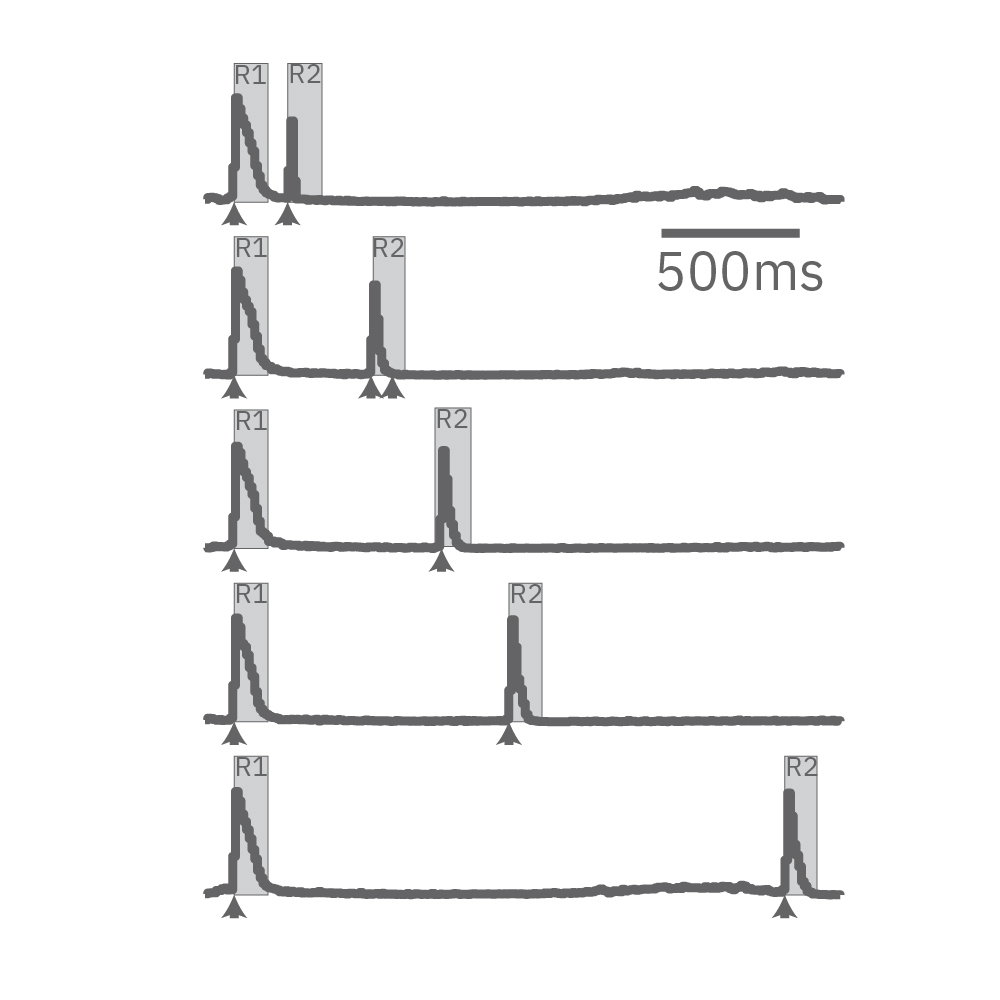
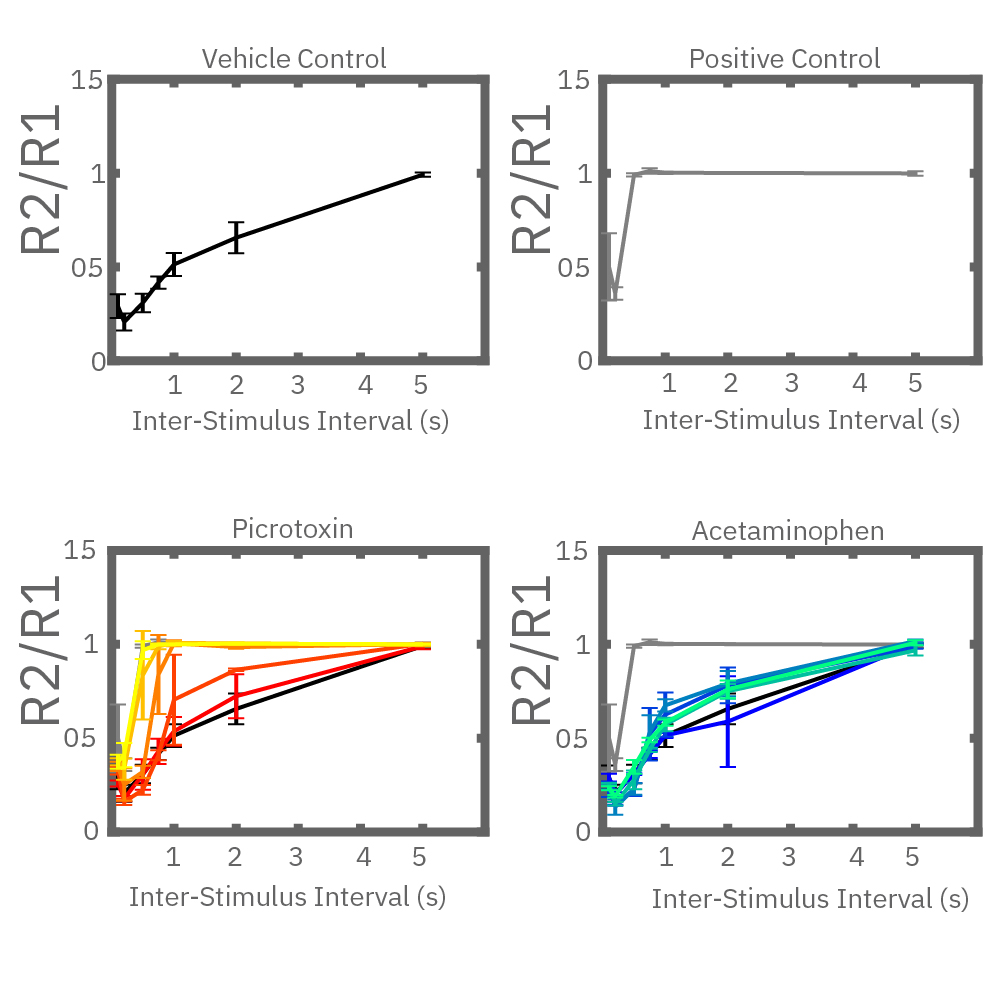
Electrical stimulation enables the computation of evoked activity metrics. For each electrode key parameters of the stimulus-evoked response can be calculated and used to assess seizurogenic activity. As an example, a “paired stimulus” assay can assess the excitatory-inhibitory balance of a network. The assay measures the ratio of the evoked response between two stimuli, with increasing delay between them. Compounds that decrease inhibition in the network, like picrotoxin, create a leftward shift in the plot.
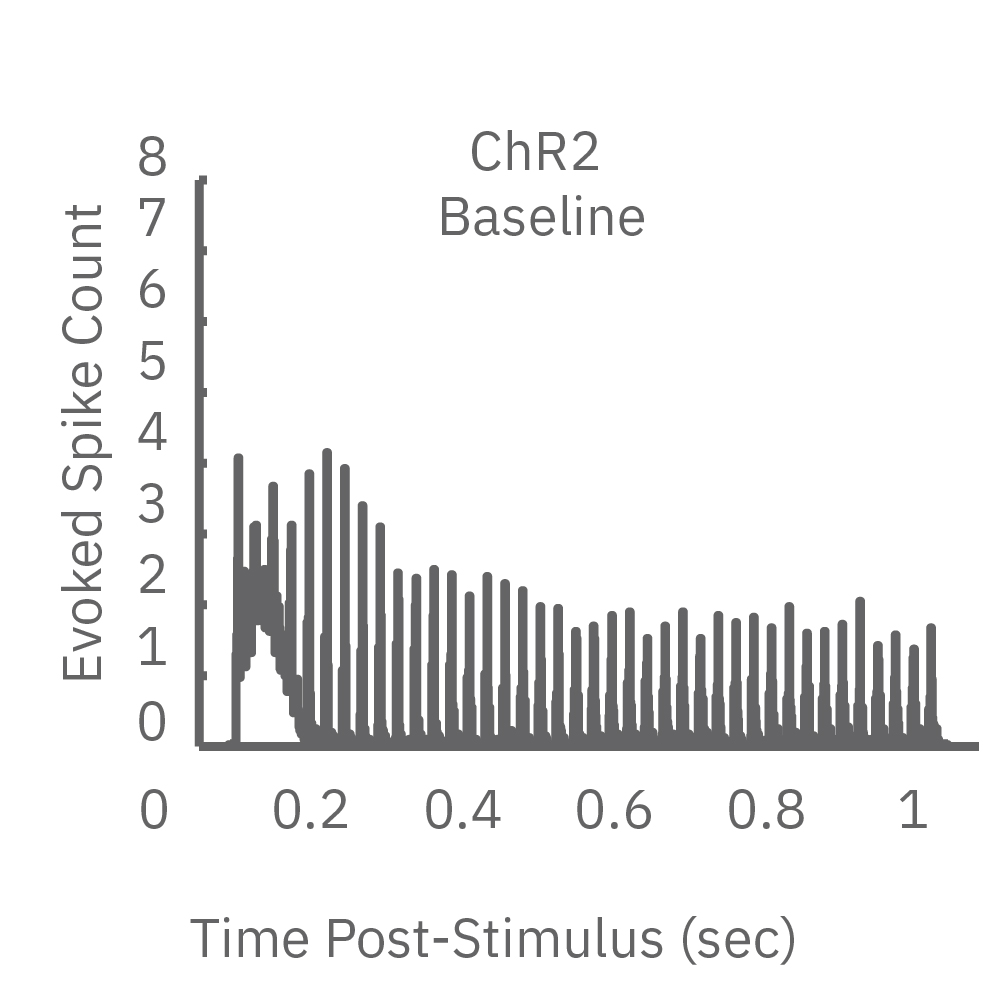
High-frequency blue light stimulation (2 ms pulses at 40 Hz for 1 s) was applied with the Lumos to channelrhodopsin-expressing (ChR2) primary rat neurons. The light-evoked neural response showed adaptation with reduced responses to later pulses in the train. In contrast, the opsin Chronos has faster kinetics which enabled a consistent response to each pulse in the high-frequency train.
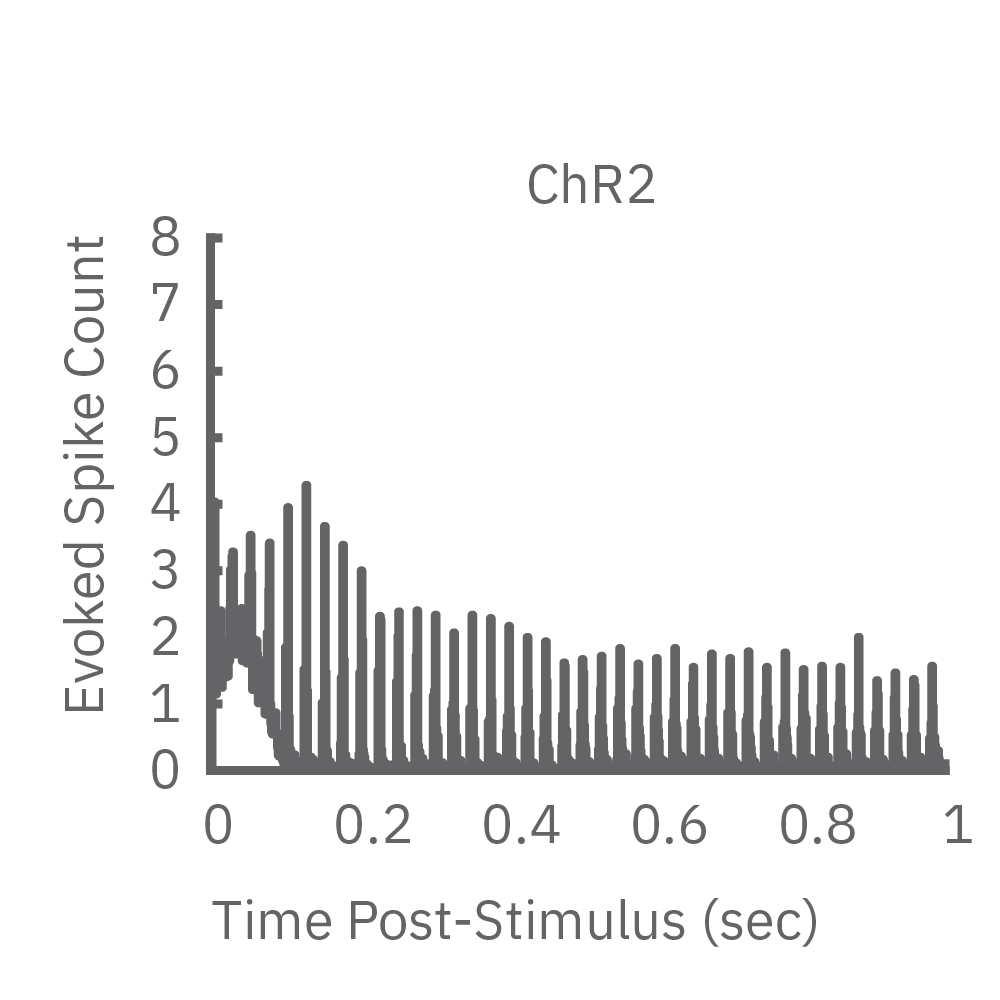
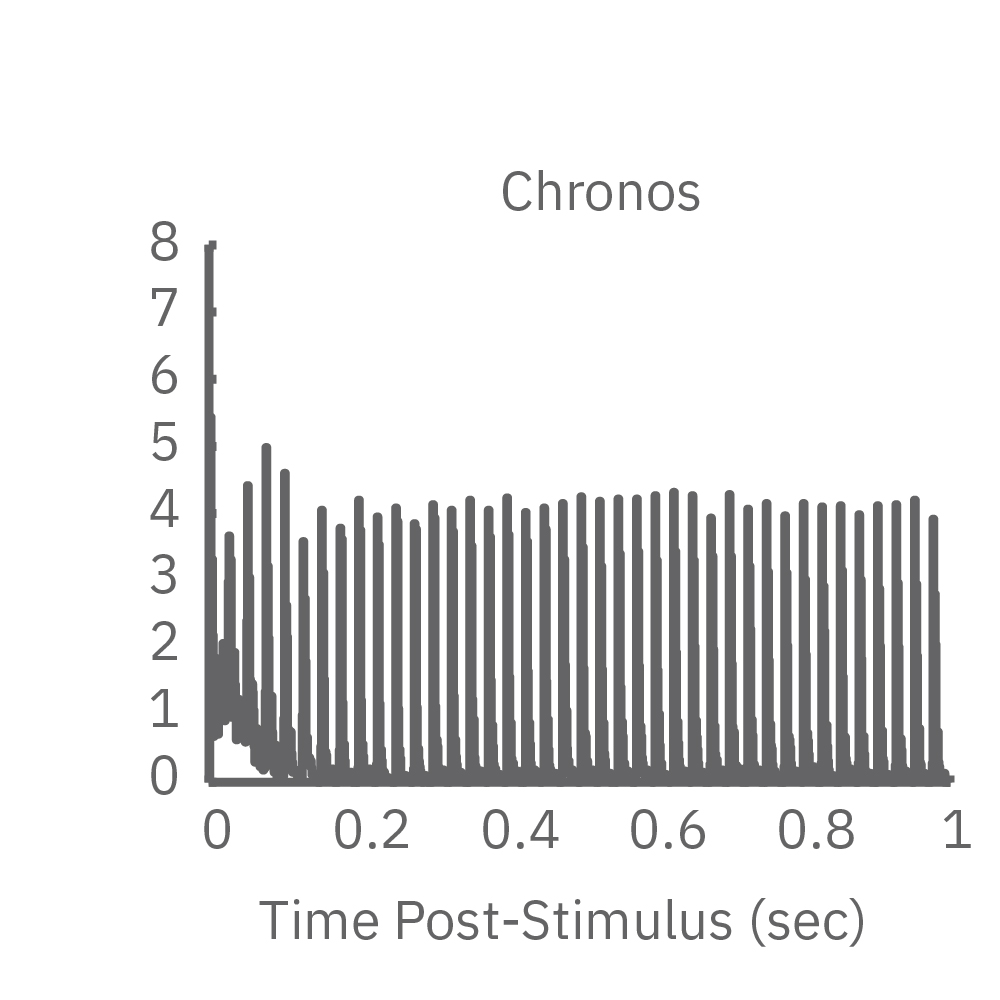
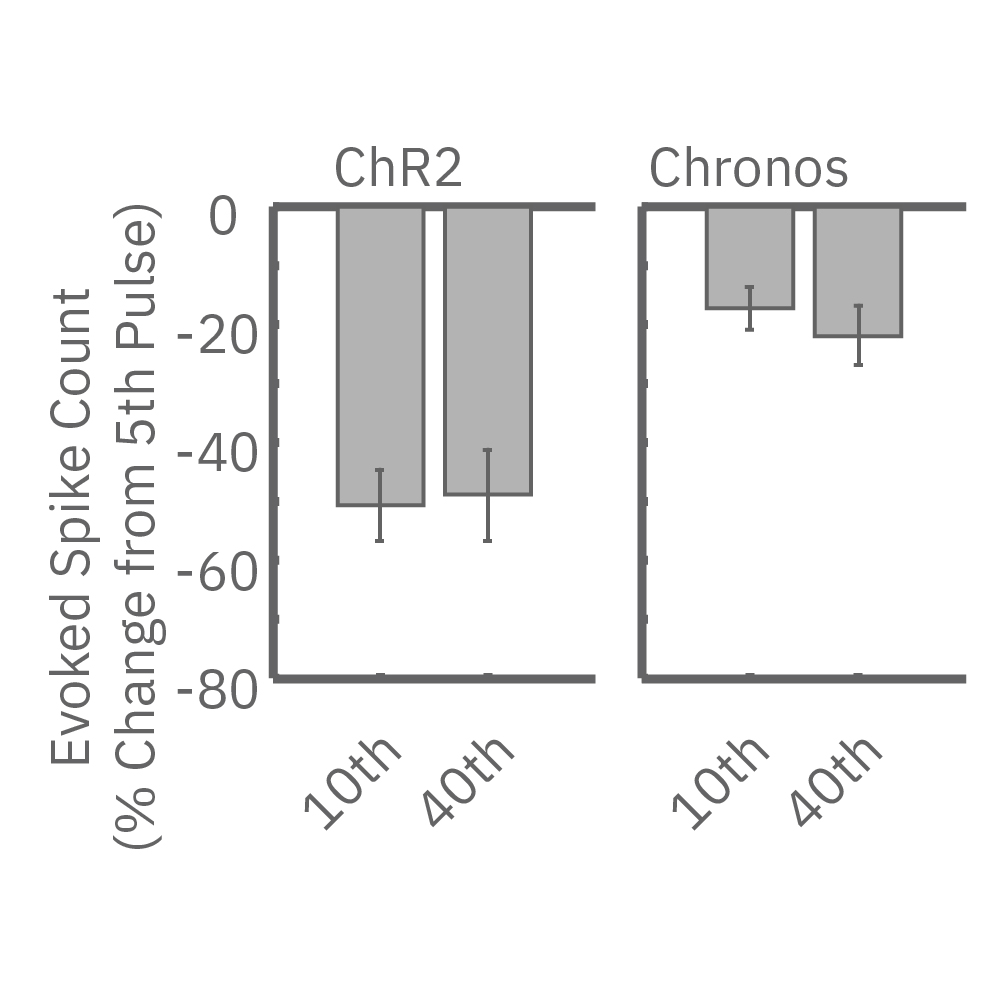
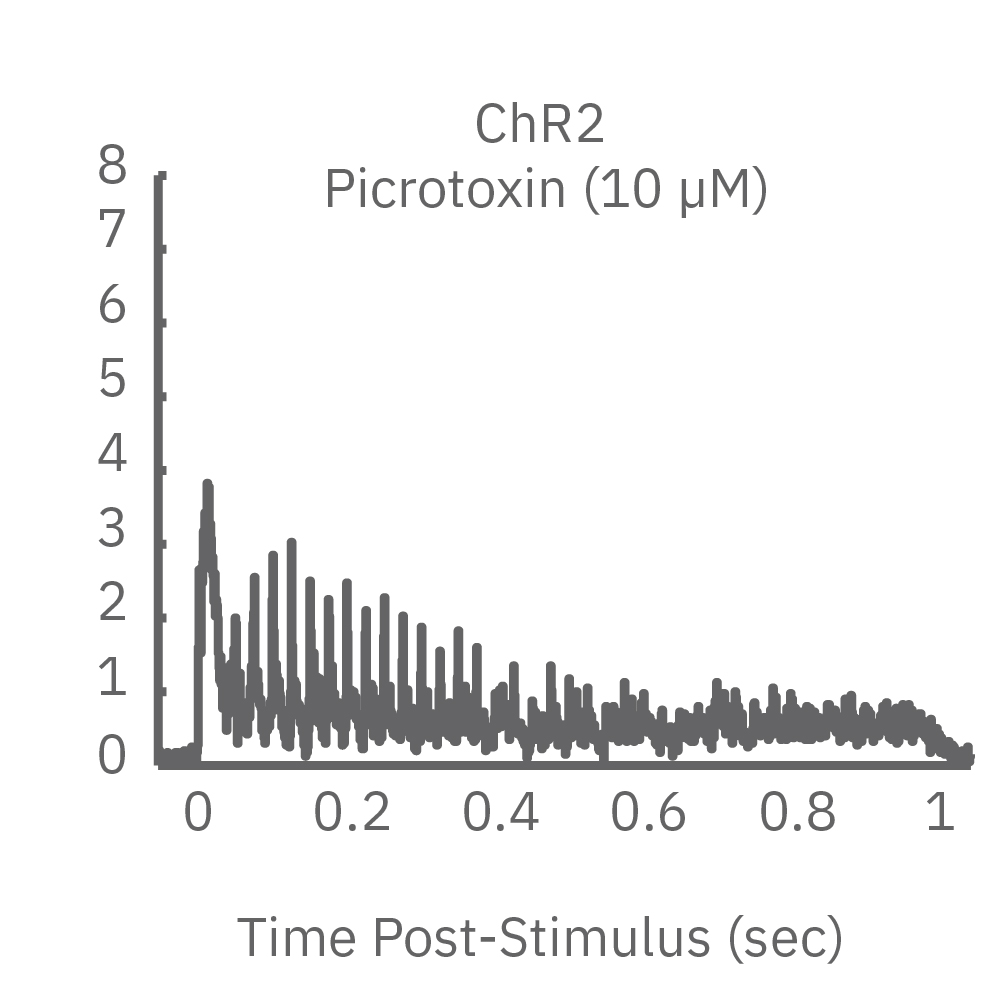
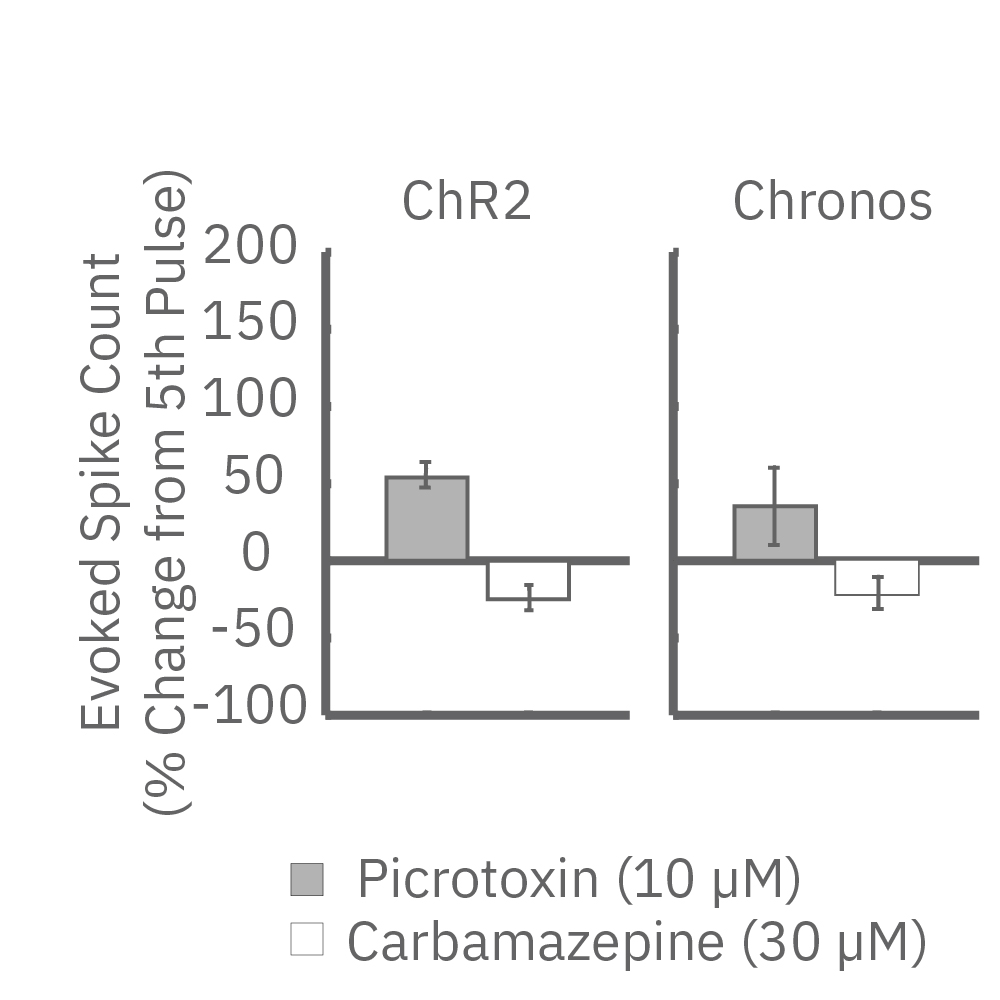
ChR2 and Chronos neurons were dosed with either a proconvulsant (picrotoxin,10µM), antiepileptic (carbamazepine, 30µM). Neural networks dosed with picrotoxin showed a sustained response to high frequency blue light stimulation (2ms pulses at 40Hz for 1s) in which firing did not return to zero between each pulse, resulting in a higher cumulative spike count across the 40 pulses. In contrast, carbamazepine reduced the response, resulting in a lower cumulative spike count.
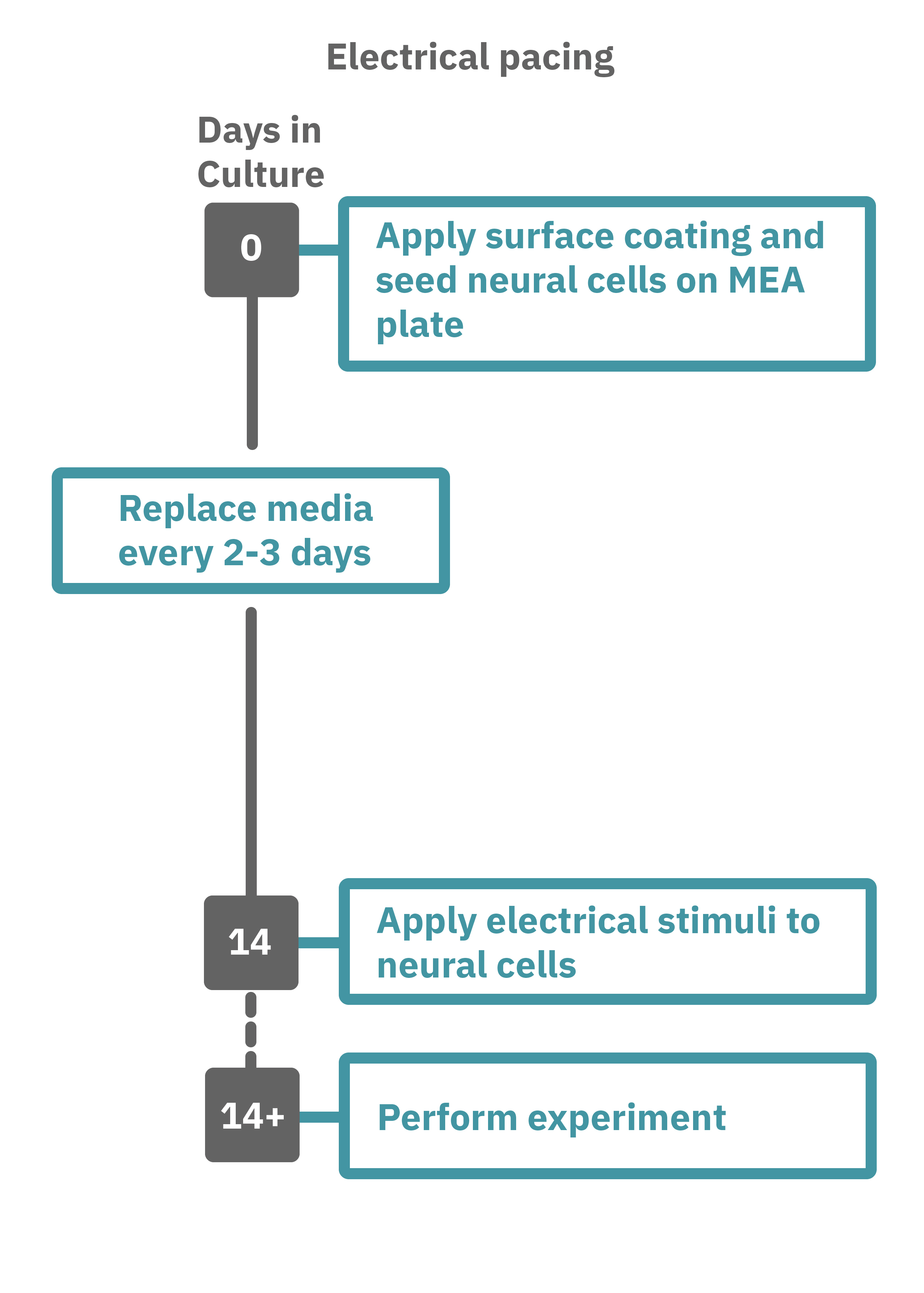
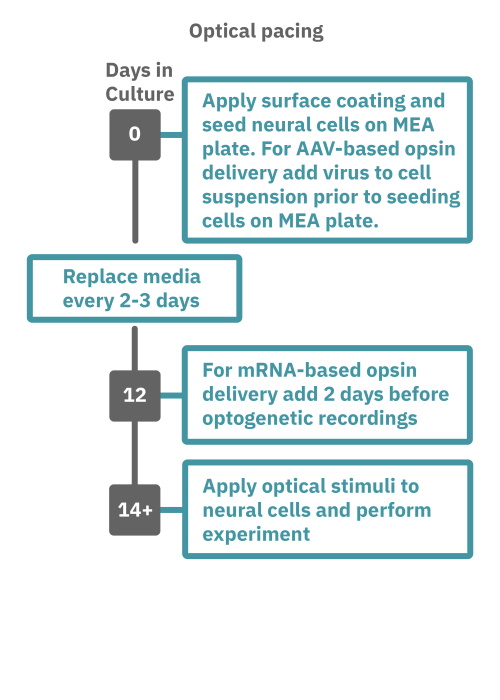
Getting started with Maestro Pro and Edge couldn't be easier. Culture your neurons in an Axion multiwell MEA plate (Day 1). For optogenetic experiments, using adeno-associated virus-based vectors, add the virus to the cell suspension at the time of plating. Allow 7-14 days for opsin expression. When transfecting neurons with mRNA-based opsins, transfect at peak neural activity and allow 2 days for opsin expression. Load the MEA plate into the Maestro MEA system at the desired recording times and begin recording. Perform optical stimulation experiments using the Lumos optical stimulator, or electrical stimulation studies using any microelectrode in the MEA plate (Day 14). Analyze the neural activity with AxIS Navigator software.