A neural co-culture is simply an in vitro culture that includes more than one type of cell. Because neurons co-exist with other types of cells in the central nervous system, such as microglia and astrocytes, neural co-cultures can better mimic the complex interactions between cells and provide a more robust platform for studying neurodevelopment, disease progression, and neural function. Dysfunction of microglia, a key component of the nervous system’s innate immune system, has been implicated in many neuropsychiatric and neurodegenerative diseases, including bipolar disorder, depression, Alzheimer’s disease, and Parkinson’s disease.
To explore neuro-immune interactions and how the Maestro is being used to investigate their impact in health and disease, download our Publication Highlights review.
Model complex neural co-culture interaction with the Maestro Pro
-
Investigate microglial and astrocyte regulation of neural activity>
-
Develop complex models of neuro-immune organoid network development>
-
Investigate microglial and astrocyte regulation of neural activity>
-
Watch the interplay of inhibitory and excitatory neurons in neural co-cultures>
-
Advantages of recording neural co-culture interactions with the Maestro Pro>
-
Publication Highlights: Neuroimmune>
Microglia continuously monitor their microenvironment and respond to pathological changes. They play a critical role in protecting the brain from runaway excitation by regulating local neural activity and acting as the resident macrophage scavengers.
By recording from co-cultures of primary cortical neurons and microglia, the Maestro MEA platform reveals the mechanisms underlying microglial control of neural activity. Read Badimon et al, Nature 2020 for more details.
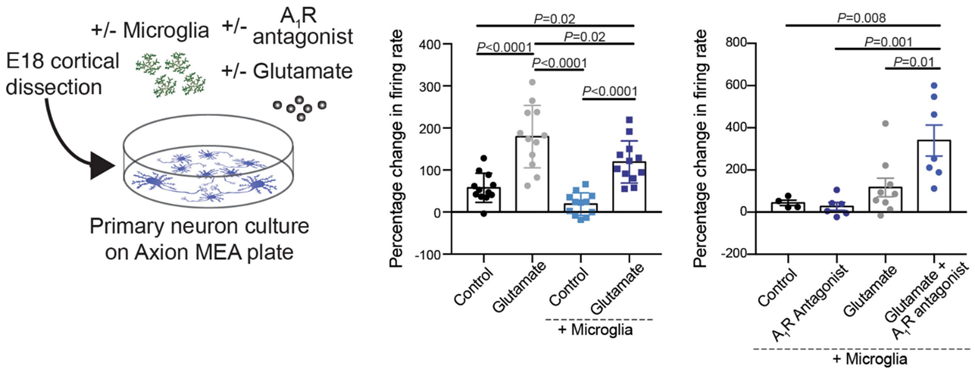
Microglia regulate neuronal activity in an adenosine receptor-dependent manner. When neurons are active, they release adenosine triphosphate (ATP), which nearby microglia convert to the neural activity inhibitor adenosine. Microglial suppression of glutamate-induced activity is blocked by an adenosine receptor antagonist. Figure modified from Badimon et al, Nature 2020.
Astroctye-neuron interactions also support homeostatic regulation of neural network activity. When co-cultured with astrocytes, iPSC-derived glutamatergic neurons show a 40% increase in network bursting (n=10 wells). After the addition of 100 nM picrotoxin, the difference increases to 84%. No activity is seen with astrocytes alone (data not shown), suggesting the astrocytes are not responsible for the activity directly, but through their influence on the glutamatergic neurons.
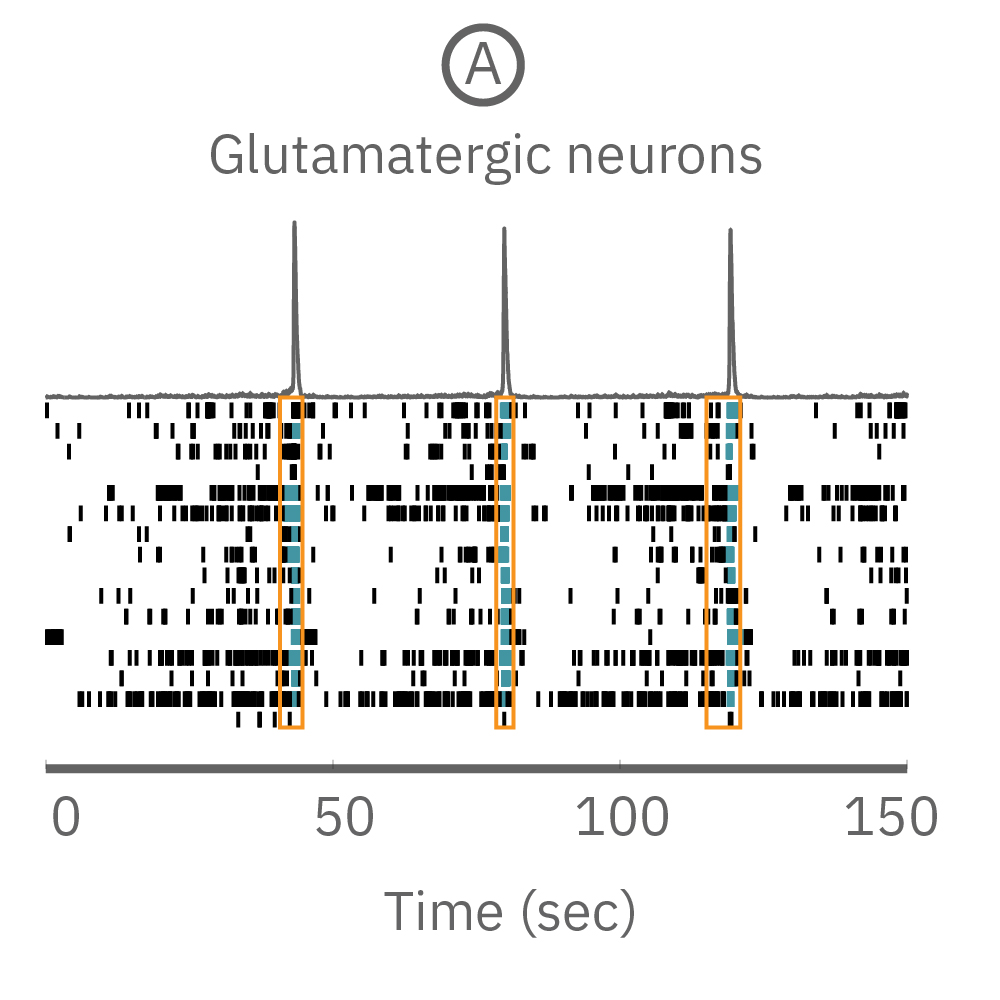
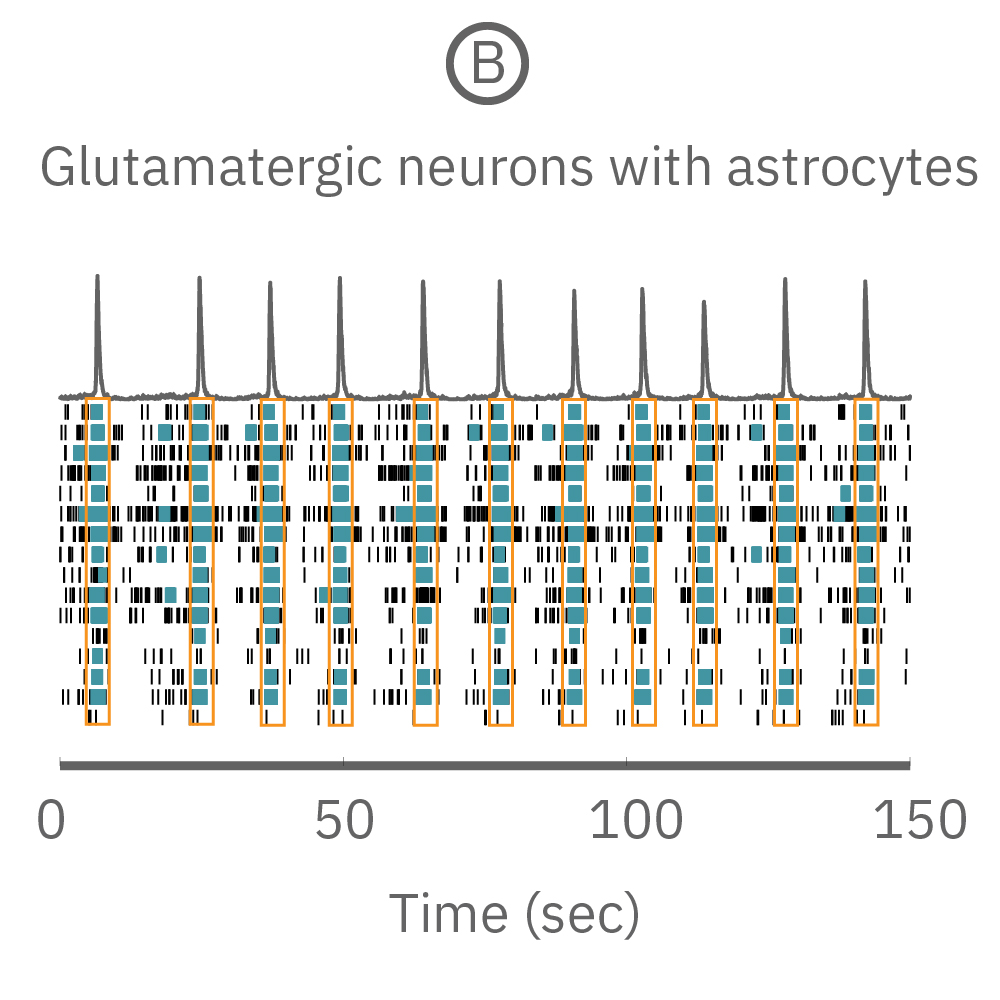
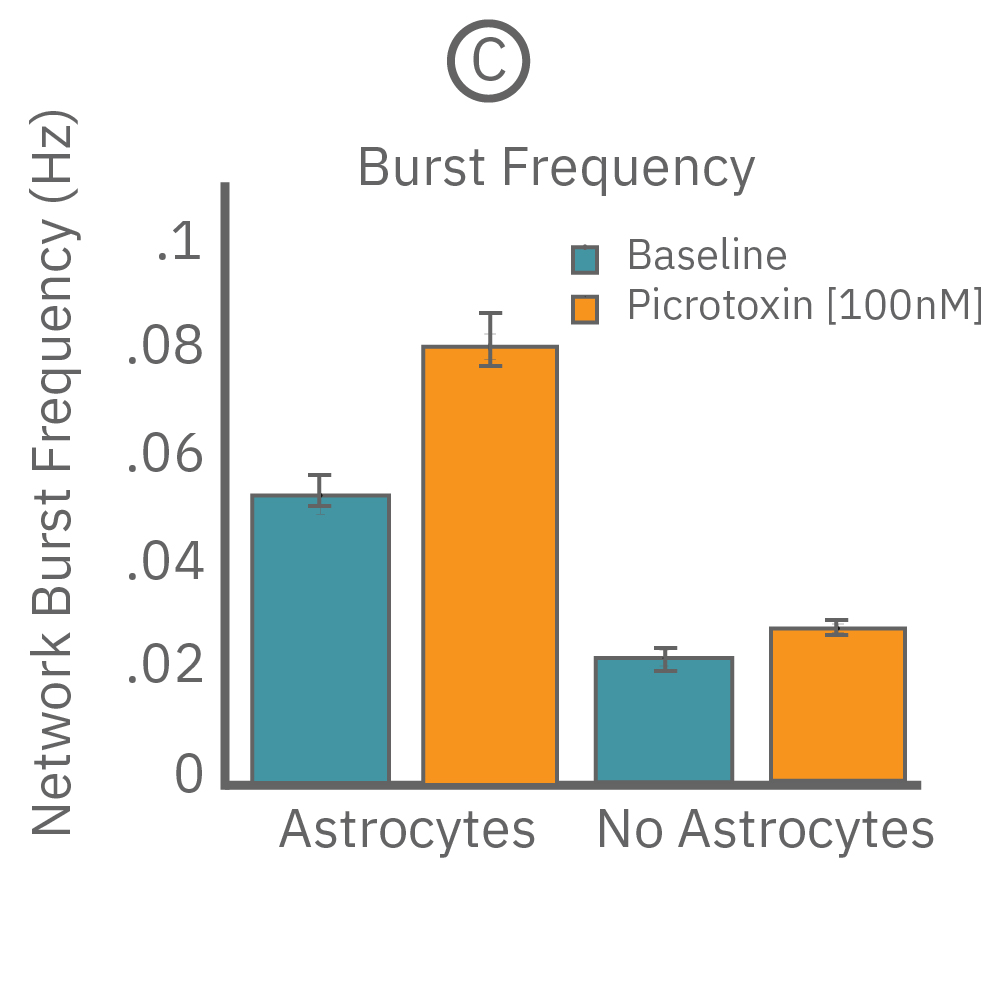
(A) A raster plot of glutamatergic neurons and (B) glutamatergic neurons with astrocytes. (C) The response of network burst frequency in both cultures to 100 nM picrotoxin.
Neuroimmune organoids offer a powerful model for studying the role of neuron-microglia interactions in neurogenesis and network development. Microglia not only protect neurons from stress and damage, they also accelerate network development. In Popova et al, Cell Stem Cell 2021 the Maestro MEA platform showed how transplanted microglia increased synchronous, oscillatory network activity of cortical neuron organoids.
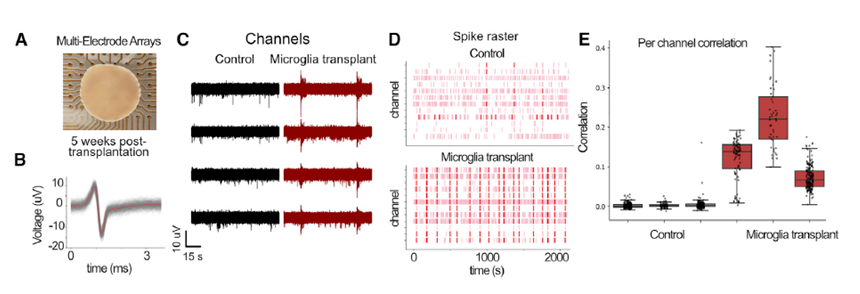
Transplanted microglia promote and accelerated the development of synchronous network activity in cortical neural organoids. Figure from Popova et al, Cell Stem Cell, 2021.
Chronic neuroinflammation can impair glial function, leading to neurodegenerative disease. In Alzheimer’s disease, inflammation reduces microglial clearance of amyloid beta (Aβ42), resulting in synaptic damage and pruning.
In Li et al, Frontiers in Immunology 2020, researchers used the Maestro MEA platform to understand how amyloid beta accumulation impacts cortical neuron function. Exposure to Aβ42 oligomers leads to hyperexcitability in vitro after only 24 hrs, mirroring the phenotype observed in vivo. Changes in biophysical properties of neurons are also confirmed using spike sorting.
Co-culture with activated, bone-derived macrophages results in synaptic preservation and phenotype rescue, again mirroring in vivo observations and suggesting a promising therapeutic.
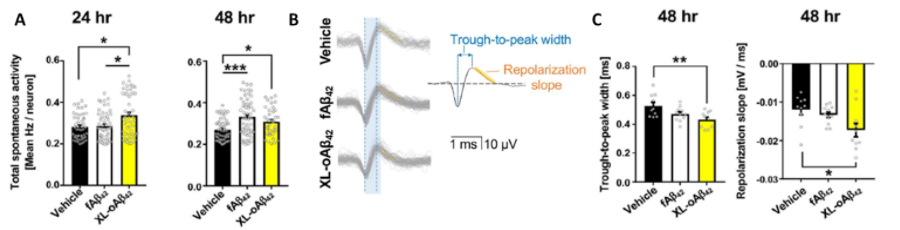
(A) Cortical neuron activity is more sensitive to Aβ42oligomers (XL-oAβ42) compared to fibrils (fAβ42) after only 24 hrs. Activity significantly increases after exposure to both fibrils and oligomers after 48 hours. (B, C) Changes in activity are accompanied by changes in extracellular spike shape, including shorter trough-to-peak width and faster repolarization. Figure modified from of Li. et al, Frontier in Immunology 2020
Observe how these interactions develop over time and how individual components can alter your disease phenotype and understanding of its progression.
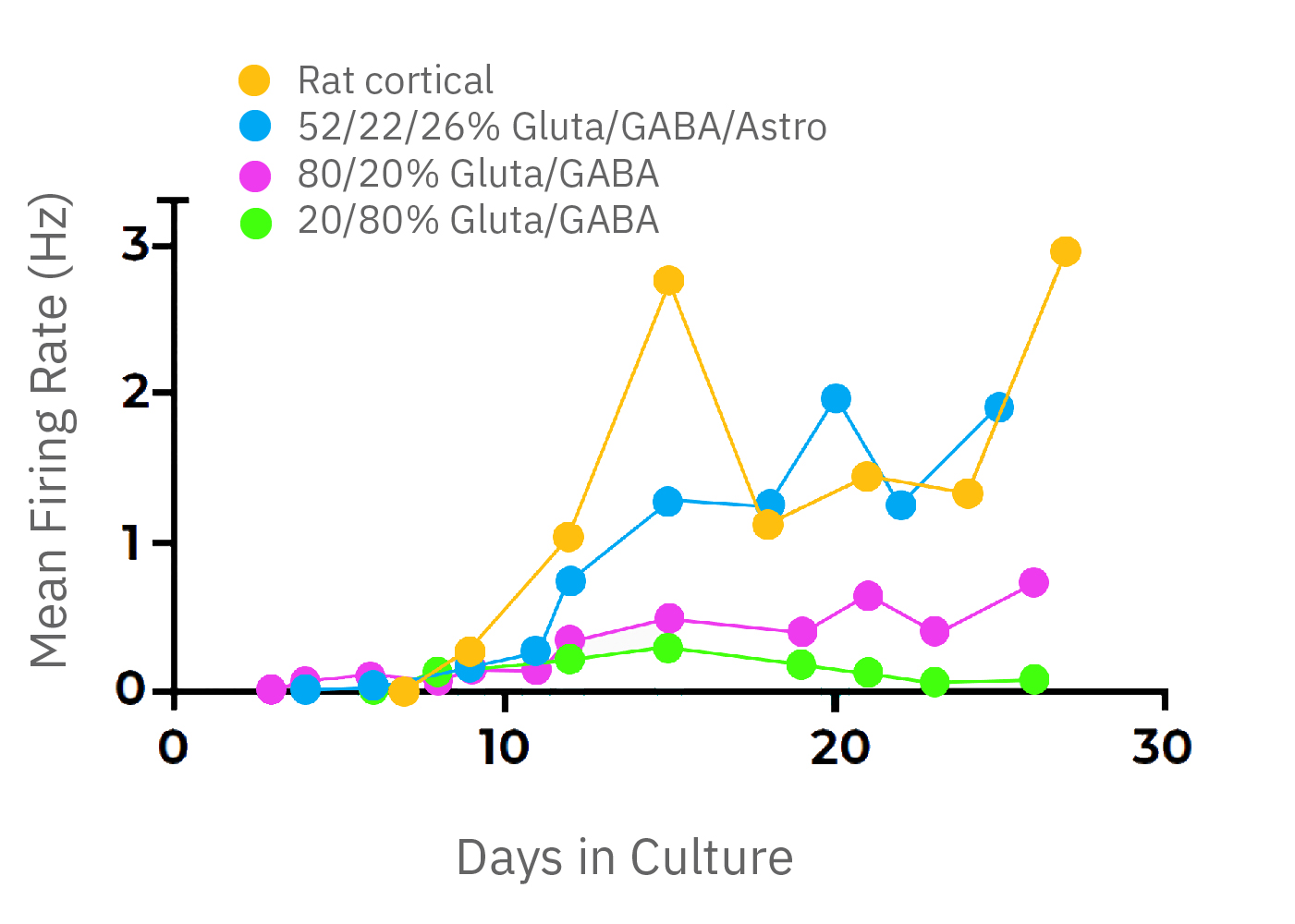
The balance of excitation and inhibition define the activity of neural circuits. Different regions of the brain contain different compositions of neuronal and glial cell types and their activity is marked by distinct network phenotypes.
With iPSC technology it is possible to customize the composition of your neural co-cultures by co-culturing known neuronal subtypes. In this example, glutamatergic and GABAergic neurons are cultured for two weeks at different proportions. The activity of cultures with predominately GABAergic populations peak around 2 weeks, but lack coordinated network activity. The large inhibitory population prevents synchronous bursts of network activity.
Network bursting is seen when the ratios are reversed, with excitatory glutamatergic neurons driving increased activity. With the addition of astrocytes, a 52/22/26% mixture (glutamatergic, GABAergic, and astrocyte) exhibits similar activity levels to primary rodent cortical neurons.
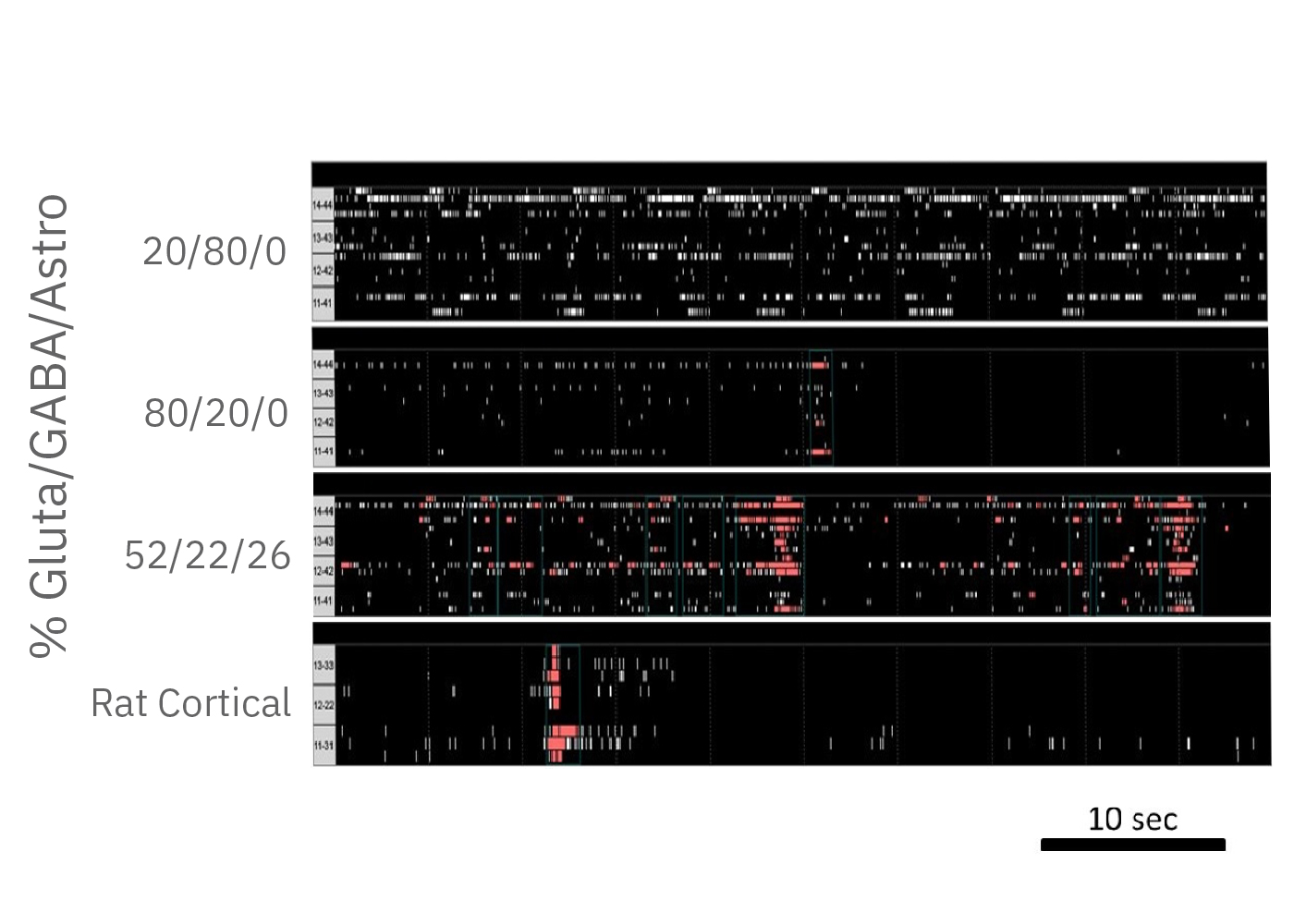
(A) A raster plot of glutamatergic neurons and (B) glutamatergic neurons with astrocytes. (C) The response of network burst frequency in both cultures to 100 nM picrotoxin.
How does your culture compare? What types of cells are present and how are they influencing your results?
- >> Understand how the composition of your culture influences its activity and optimize for more of an in vivo phenotype.
- >> Discover the role of microglia and astrocytes in neurodegenerative and neuropsychiatric disease.
- >> Chart network development and disease progression simultaneously across 96 cultures.
- >> Selectively target specific neuronal subpopulations with the Lumos optogenetic stimulator or electrically stimulate side-by-side cultures within the same well using the Culture-Insert 2 Well (ibidi, 80209).

Publication Highlights: Neuroimmune
The neuroimmune field is always evolving. Download highlights from four publications by researchers using the Maestro platform to understand the neuronal and immune cell effects on network activity.
“The MEA system is a great addition to our lab and has expanded our studies.”
Our lab has benefitted significantly by combining the power of the Maestro MEA system and AxIS software, with our human stem cell-derived neuron-glial cell cultures. We are now able to follow, in real time, the development and functional maturation of neurons, glia, and neural circuits, not only over weeks but over years. We can ask new scientific questions that, previously, were difficult or impossible to answer. In some of our most recent experiments, we were using the MEA system to record from human neurons maintained in culture for almost 2 years. We believe this may be a record for 2D human neuroglial cell cultures.
- Robert Halliwell. University of the Pacific School Of Pharmacy, California, USA








