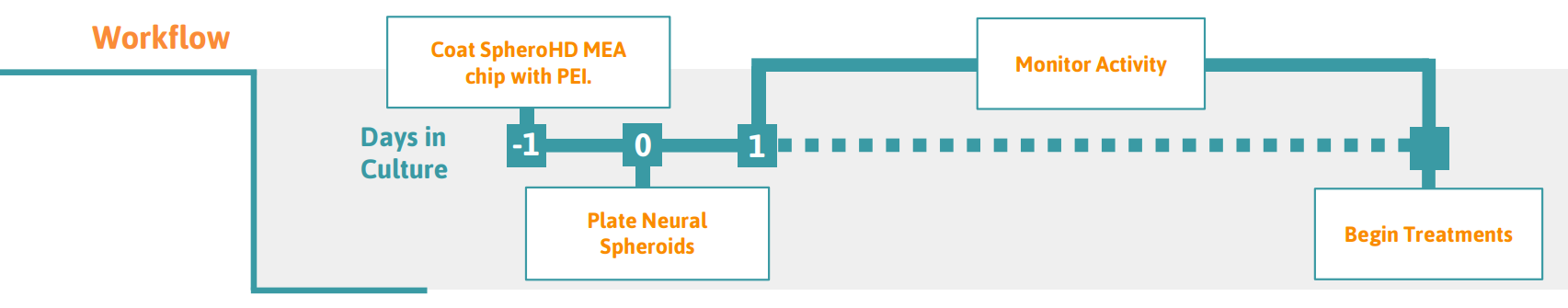
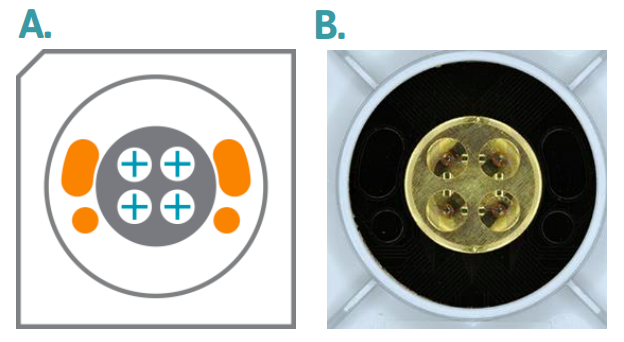
Preparing the SpheroHD Chip
- Pipette 10 μl of 0.1% PEI in borate buffer (or neural coating of choice) into each of the four chambers of the Ibidi insert on the SpheroHD chip.
- Incubate the PEI-coated SpheroHD chip in a cell culture incubator at 37°C, 5% CO2 for at least 60 minutes.
- Aspirate PEI from the chambers and rinse with 10 µl of sterile DI water 4 times, then allow the SpheroHD chip to air dry in a biosafety cabinet overnight at room temperature.
Culturing and Plating Neural Spheroids
Add 10 µl of neural spheroid media to each chamber of the Ibidi insert on the SpheroHD chip.
Tip: More information on proper handling of the Ibidi 4 well FulTrac mico insert can be found on Ibidi’s website (Cat. No: 80486).
Centrifuge at 1500 x g for 1 minute using the provided single-well centrifuge holder to remove bubbles. Remove the media from each chamber.
Tip: Transferring spheroids in small volumes (10 µl, e.g.) can be difficult. Alternatively, spheroids can be transferred in larger volumes by pipetting up the spheroid, allowing it to fall to the bottom of the wide bore tip, and touching the tip to media in the chamber to transfer the spheroid.
- Using a 200 µl wide-bore pipette tip, add one neural spheroid from culture to each chamber of the Ibidi insert on the SpheroHD.
Centrifuge at 100 x g for 1 minute using the provided single-well centrifuge holder to bring the spheroids to the bottom of the chambers. Check under the microscope to ensure that all spheroids are positioned over the electrodes at the bottom of the well. Repeat or increase speed if necessary.
Tip: Some organoid/media compositions may require centrifugation to attach to the bottom of the plate. We recommend starting at 50 x g for 30 seconds and optimizing speed and time as needed.
- Once the spheroids are in place, incubate the SpheroHD MEA chip in a cell culture incubator at 37°C, 5% CO2 for 1 hour.
- Add enough neural spheroid media (about 1.75 mL) to cover the top surface of the Ibidi insert. Maximum volume for the SpheroHD chip is 2 mL.
- Take recordings using the MEA Creator Kit on the Maestro Pro, Edge, or Volt as desired.

Required Materials
Consumables
| Item | Vendor | Catalog # |
| Axion SpheroHD MEA Chip | Axion BioSystems | |
| Neural Spheroid Media | Various | |
| 50% Polyethylenimine solution (PEI) | Sigma-Aldrich | P3143 |
| Sterile DI water | Thermo Fisher | 14040 |
Equipment
| Item | Vendor | Catalog # |
| Maestro Pro MEA System | Axion BioSystems | |
| MEA Creator Kit | Axion BioSystems | |
| AxIS Navigator | Axion BioSystems | |
| 37°C Water Bath | Various | |
| Cell Culture Incubator | Various | |
| Biological Safety Cabinet | Various | |
| Tabletop Centrifuge | Various | |
| Phase Contrast Microscope | Various |





