Rockley KL, Roberts RA and Morton MJ
ApconiX, Alderley Park, Alderley Edge, Cheshire, UK
BTS Annual Congress 2022 Poster
Introduction:
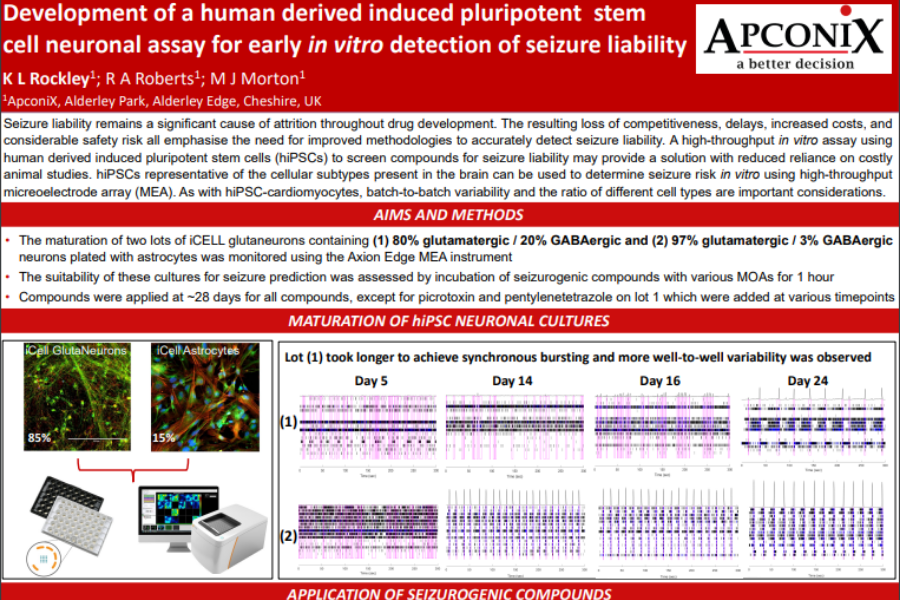
Seizure liability remains a significant cause of attrition throughout drug development. The resulting loss of competitiveness, delays, increased costs, and considerable safety risk all emphasise the need for improved methodologies to accurately detect seizure liability. A high-throughput in vitro assay using human derived induced pluripotent stem cells (hiPSCs) to screen compounds for seizure liability may provide a solution with reduced reliance on costly animal studies. hiPSCs representative of the cellular subtypes present in the brain can be used to determine seizure risk in vitro using high-throughput micreoelectrode array (MEA). As with hiPSC-cardiomyocytes, batch-to-batch variability and the ratio of different cell types are important considerations
Thank you to the authors for sharing their presentation.