Author: Jim Ross, Ph.D.
Bioelectronic assays allow scientists to precisely and continuously monitor cultured cells without labels or dyes. Dr Jim Ross, Chief Technology Officer and co-founder of Axion Biosystems, elaborates.
Young industry, massive momentum
Although cell-based immunotherapies for cancer treatment are relatively new, they are poised to take the biomedical world by storm. Kymriah was the first cell therapy approved by the US Food and Drug Administration (FDA) in 2017 for the treatment of refractory B-cell acute lymphoblastic leukaemia1 followed by Yescarta and Tecartus for large B-cell non-Hodgkin lymphoma and mantle cell lymphoma, respectively.1 Embracing the widespread adoption2 of these treatments, the US government has set an ambitious goal to start approving 10 to 20 new cell therapies per year by 2025.3 Researchers are rapidly developing new cell therapies to fuel this market growth: as of March 2020, cancerresearch.org identified 677 cell therapy trials – a marked increase from 2015, when it listed 78.1 In parallel, researchers are seeking ways to improve manufacturing efficiency, improve safety, and reduce costs to bring these therapies to market – all while working within current and anticipated government regulations.
Immunotherapies empower patients’ immune systems to combat deadly cancers that were previously intractable. They are made from different kinds of immune cells, the most common being the chimeric antigen receptor (CAR)-T cell variety. However, several other types of immunotherapies are promising alternatives, including CAR natural killer (CAR-NK) cell therapy, which is rapidly gaining attention in the field due to its potential to offer a safer, less expensive, and more effective option.4,5
Immunotherapies leverage existing biological processes to produce a highly personalised medical intervention. Because these therapies involve living components, some steps of the manufacturing process are not entirely under the manufacturer’s control. To make a CAR-T therapy, T cells are harvested from the patient’s – or, in rare cases, a donor’s – blood and then expanded and transfected with a virus that genetically modifies them to produce a CAR transgene that targets the patient’s tumour.6 Similarly, CAR-NK therapies are produced by harvesting mononuclear cells from blood and modifying them to produce a CAR transgene.7 CAR-NK manufacturing is faster, more efficient, and allows for safer delivery via donor cells; however, this class of cell therapy has been difficult to engineer, and often displays low levels of gene expression.8 In both cases, variability in the manufacturing process leads to variability in safety and potency – i.e. how well the therapy kills cancer cells. Therefore, before cells are infused back into the patient, scientists must test each batch to ensure the cells are safe and will deliver the correct dose of therapy.
Navigating the somewhat unpredictable nature of the development and manufacturing process has led to a high cost of production for current cell therapies. Significant safety and toxicity concerns involving off-tumour effects and variations in potency are the main focus, as these can lead to life-threatening hyperactivation of a patient’s immune system. Scientists are working to develop cost-effective methods to address these concerns that also raise the standard in the field. Flow cytometry has traditionally been used to characterise batches of a cell therapy based on the number of cells producing the desired surface proteins, but a flow cytometry-based potency assay provides only a single endpoint. Therefore, there’s a growing focus in the cell therapy field to find better ways to characterise potency more precisely.
For cell therapy development, an ideal potency assay should quantify immune cell-mediated killing at physiologically relevant cell ratios to provide insight into each candidate’s functionality. It should generate kinetic data to enable researchers to test and rank candidates, not only based on cytotoxicity at an endpoint, but also how that cytotoxicity manifests over time. Such a potency assay could also inform manufacturing, where careful measures to monitor potency and establish a delivery protocol are essential to ensure that each patient receives the right dose.
Endpoint reactions to measure potency
Historically, endpoint methods have been used to characterise CAR-T and CAR-NK cells based on potency. These reactions run for a pre-determined length of time and produce a snapshot of the experiment. Several common endpoint methods include chromium release assays, colorimetric lactate dehydrogenase (LDH) assays, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays.9 Each of these involves combining the cell therapy with cancer cells and quantifying a specific chemical to mark cell death.
The chromium release assay has traditionally been the most commonly used endpoint method for measuring cytotoxicity.10 To start the assay, target cells loaded with chromium are exposed to active CAR-T or CAR-NK cells. At the end of the reaction, the supernatant of the cell culture is evaluated for a radioactive signal which is proportional to the number of target cells destroyed. While this assay is a longtime gold standard, some labs are shifting away from it in favour of powerful and sensitive assays that do not use harmful radiation.
LDH and MTT assays are two such non-radioactive alternatives. In both assays, when target cells are destroyed by immune cells, they produce a colour change that can be detected by a plate reader. Although colorimetric assays do not expose scientists to harmful radiation, they – like chromium release assays – are time-consuming and require considerable technical skill to carry out. Furthermore, endpoint reactions, by their nature, only measure once and cannot provide information on the kinetics of cytotoxicity without multiplying costs and consumables for each additional endpoint. As such, they may miss critical events during the assay, such as shifts the kill curve due to donor or manufacturing variability or if immune cell killing capacity becomes exhausted, allowing the cancer cells to grow again.
Real-time imaging to measure potency
Real-time methods to measure potency offer that vital layer of additional information that is lost to endpoint methods. They record not just how many cancer cells a CAR-T or CAR-NK therapy can kill in a given period, but also the kinetics of the process. Does the rate of cell killing change over the course of the assay? When is the maximal response across donors? At what point do the immune cells become exhausted and stop killing? Real-time assays can capture this information and more.
Live-cell imaging, for example on Sartorius’s Incucyte platform11, can be done under controlled conditions over days to months, giving scientists the opportunity to observe cytotoxicity as it is happening between individual cells. Live-cell assays are instrumental when optimising protocols and examining the effects of variables that are unpredictable or unknown. However, live-cell imaging relies on optical techniques that generate technical challenges for scientists monitoring the activity of an immunotherapy.
During these assays, the engineered immune cells are physically plated on top of tumour cells; depending on the opacity of the top layer, visibility may be occluded. Researchers use dyes to differentiate between the engineered cell and target tumour cells, but this adds an additional step to the workflow that further manipulates the cells being studied. Analysing imaging data can be complex, often requiring a trained eye and forcing scientists to establish subjective decision points.
A major strength of live-cell assays are that they enable researchers to observe a cell therapy in action, from start to finish. This capability is particularly valuable in the early stages of development, when many questions need to be answered at once: which antigen to target, how do we optimise CAR transduction, does expansion or cryopreservation reduce potency? But even after these questions are addressed, a good live-cell assay can detect and quantify differences in donors and manufacturing batches. To streamline efforts, reduce cost, and improve success; a desirable method should be suitable for screening, fast to set up, automate, and provide clear actionable data.
Bioelectric assays for measuring immune cell potency
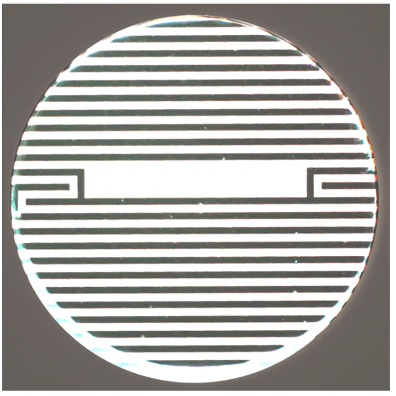
Bioelectric assays allow researchers to monitor immune-cell mediated killing of tumour cells non-invasively in real time without relying on optics, labels, or dyes of any kind. They offer objective data, which makes it easier for scientists to quickly and accurately measure cytolysis. These assays are carried out in microtitre plates with electrodes embedded in the bottom of each well (Figure 1).12
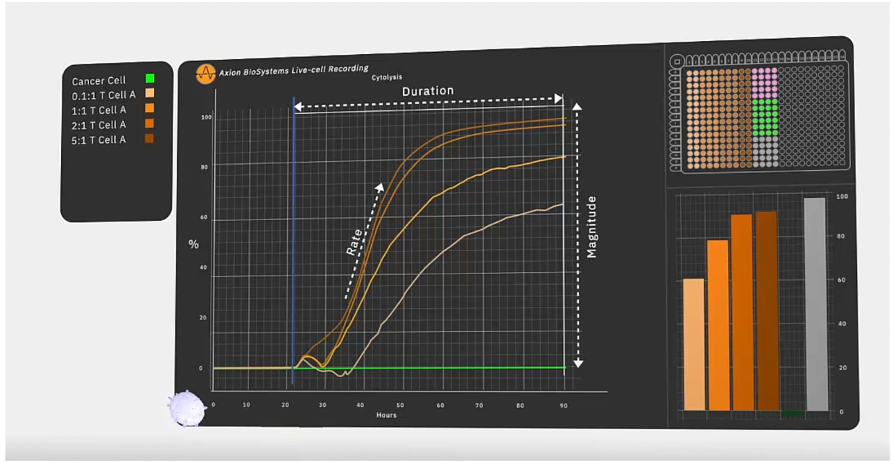
Impedance-based systems monitor cell viability and state based on the cells’ adherence to the plate. Live cells attach themselves to the well and cover more of the electrode, with an increase in impedance proportional to the number of viable cells present. As cells die, they detach from the surface, reducing impedance. In practical terms, when evaluating immunotherapies, this assay can be used to monitor the magnitude and kinetics of cell death across a range of cell concentrations to establish a precise and objective measurement of a specific therapy’s potency. (Figure 2.)
Dr Lohitash Karumbaiah and Dr Meghan Logun at the University of Georgia put this technique into practice while developing a cell therapy for glioblastoma, an aggressive type of brain cancer with no established treatments. 13 They and their colleagues monitored the cytotoxicity of GD2-targeted CAR-T cells toward glioma stem cells using bioelectronic assay plates run on THE Axion BioSystem’s Maestro Z system. The Maestro Z hosts integrated environmental controls, automatically maintaining temperature and CO2 levels set by the user, which allowed the researchers to continuously monitor cells over nine days.
In the study, the researchers collected glioma stem cells from participating patients. They added the cells to a 96-well MEA plate, allowing them to adhere for two days. Then, after adding CAR-T cells in proportions ranging from 0.1:1 to 10:1 T cells to tumour cells, the researchers observed the cell behaviour for seven days. They saw that CAR-T cells led to glioma cell death, as indicated by a decrease in impedance over time.
The researchers also took advantage of the continuous monitoring capabilities of their bioelectric assay to track the kinetics of cytolysis as a measure of the CAR-T cells’ potency. They looked at the amount of time it took for each concentration of GD2 CAR-T cells to kill 50% of the tumour cells and saw that the CAR-T cells killed over 50% of target cells by the end of the experiment, even at the lowest ratio of 0.1:1, a marked increase over the control (naïve T cells). The glioma cell killing, however, never reached 100% and flow cytometry at one and seven days confirmed what was observed on the Maestro Z, that cells at later time points showed several markers of chronic activation and early exhaustion: reducing effectiveness at killing tumour cells. Overall, the real-time bioelectric data generated by the Maestro Z demonstrated that GD2 is a promising therapeutic target for glioblastoma and when combined with more traditional assays like flow cytometry, can elucidate important therapeutic factors to consider.
Working with government regulators
Cell therapy developers and manufacturers must stay forward-looking to remain GxP compliant as the field takes off. This includes ensuring that facilities, personnel, materials, equipment, documentation, logistics, and quality assurance are all in line with evolving government regulations. FDA and other regulatory agencies are going to start wanting more potency information on cell batches, and experts in the pharmaceutical field are expecting that this data will be required for new cell therapies very soon. To remain in good standing and ensure that the cell therapies under development can proceed seamlessly with a suitable protocol, companies should proactively choose equipment that will align with the FDA’s anticipated needs.
Currently, Axion BioSystems offers the only label-free, live-cell, screening platform for GxP compliant immunotherapy potency evaluation.14 Its Maestro Z system, running the GxP impedance module, streamlines assays, while a built-in software test verifies that all results are accurate. The instrument produces a continuous data stream that is timestamped and matched to barcoded plates. For each assay, the software produces a record that is easy to validate and audit to meet regulators’ needs and enable advanced analysis.
Conclusion
The Maestro Z offers GxP compliant, noninvasive, real-time measurements to assess the quality of a cell therapy, making it an excellent addition to an immunotherapy lab. Tracking potency allows scientists to gauge the robustness of their manufacturing workflow and helps predict the efficacy. As the immunotherapy field develops and optimises methods to evaluate new cell-based therapies, researchers and industry leaders must continue working with government agencies to establish guidance and regulations that ensure the quality and safety of this new class of therapeutic products. They are in a prime position: regulations are still being shaped as fast as new therapies are being developed. As such, immunotherapy experts have the opportunity to embrace technologies they’ve found to be best suited to overcome challenges they’ve encountered, to offer cell therapies that are increasingly safe, effective, and accessible to patients that need them.
About the author

Dr Jim Ross is the Chief Technology Officer and co-founder of Axion Biosystems, a company that develops instrumentation to support Bioelectronic assays in the life sciences. Bioelectronic assays allow scientists to precisely and continuously monitor cultured cells without labels or dyes.
References:
- Hanover L. The Untapped Potential of Cell and Gene Therapy. Evidence-Based Oncology (2021) 27(2): SP51
- Find a Kymriah treatment, https://www.us.kymriah.com/treatment-center-locator/
- Statement from FDA Commissioner Scott Gottlieb, MD, and Peter Marks, MD, PhD, director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies, https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics. Accessed January 13, 2021.
- Yu JX, Upadhaya S, Tatake R, Barkalow F, et al. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov. 2020;19(9):583-584.
- No authors listed. Natural killer cells for cancer immunotherapy: a new CAR is catching up. EBioMedicine. (2019) 39:1-2.
- Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. (2017) 5:22.
- Liu, S., Galat, V., Galat4, Y. et al.NK cell-based cancer immunotherapy: from basic biology to clinical development. J Hematol Oncol (2021) 14, 7.
- Schmidt P, Raftery MJ, Pecher G. Engineering NK Cells for CAR Therapy-Recent Advances in Gene Transfer Methodology. Front Immunol. (2021) 11:611163.
- Fotakis G, Timbrell JA. In vitro cytotoxicity assays: comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol Lett. (2006) 160(2):171-177.
- Wonderlich J, Shearer G, Livingstone A, Brooks A. Induction and measurement of cytotoxic T lymphocyte activity. Curr Protoc Immunol. (2006) Chapter 3.
- Smarter Technologies for Advancing the Next Generation of CAR-T Therapies, https://www.sartorius.com/en/applications/life-science-research/cell-biology/cell-therapy-research/car-t-research
- Axion Biosystems. Technology, https://www.axionbiosystems.com/about-us/technology
- Chvatal S, Logun M, Hayes H, et al. Abstract 2187: Kinetics and potency of T Cell-mediated cytolysis of glioblastoma. In: American Association of Cancer Research, editor. Proceedings: AACR Annual Meeting 2020 [Internet]; 2020 April 27-28 and June 22-24; Philadelphia, PA. Philadephia: Cancer Res, 2020.
- GxP Impedance Module, https://www.axionbiosystems.com/products/software/gxp-impedance-module
Related Products
There are currently no products tagged to this resource.