In vitro cardiac safety assays with the Maestro™
The Comprehensive in vitro Proarrhythmia Assay (CiPA) initiative seeks to redefine preclinical cardiac safety assessment for new drugs. Previous regulations focused on the blockade of the human Ether-à-go-go related gene (hERG) potassium channel, resulting in QT prolongation and Torsades de Pointes. While highly sensitive, this approach lacks specificity, leading to a high drug attrition rate. CiPA aims to increase predictivity of drug safety assessments, with in vitro evaluation of whole cell drug effects on human stem cell-derived cardiomyocytes (hSC-CM) playing a critical role. Specifically, hSC-CMs provide an integrated assessment of multiple ion channel effects which may compensate for hERG block.
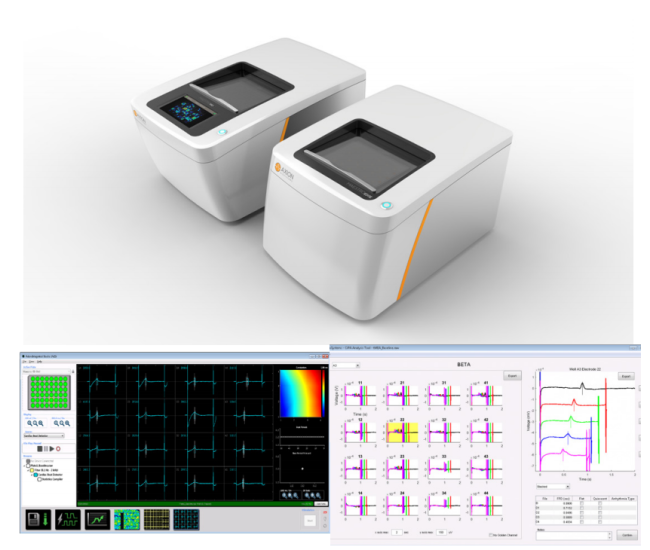
Axion BioSystems’ Maestro™ multiwell MEA platforms are well-positioned to evaluate complex hSC-CM electrophysiology with accessible high-throughput tools. The Maestro platforms offer an industry-leading 768 electrodes for precise and efficient evaluation of key cardiac electrophysiology endpoints at scale. The Maestro Pro and Edge integrate seamlessly with AxIS software and offline tools to make analysis and standardized reporting quick and intuitive. These automated analysis tools extract measures of repolarization and arrhythmia, while also allowing for user visual inspection and verification.
The Maestro Advantage
>> Label-free, non-invasive voltage recording enables long-term monitoring of cardiomyocytes
>> Industry-leading number of electrodes (768) provides high quality spatiotemporal resolution
>> Environmental controls provide a stable environment for both acute and chronic studies
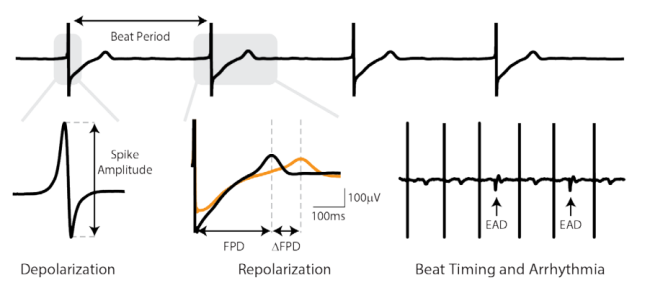
>> Fast data collection rate (12.5 kHz) accurately captures depolarization spike amplitude
>> Scalable format (12-, 48-, and 96-well plates) allows throughput flexibility all on one system
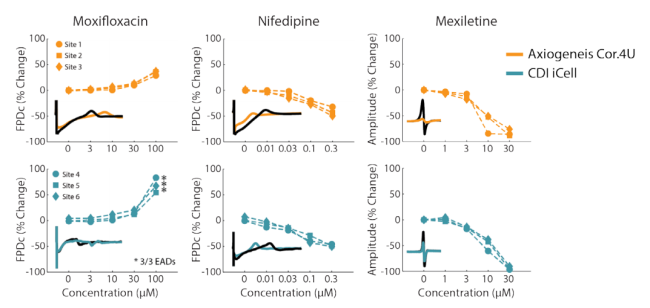
Highly reproducible across sites and cell types
In the CiPA pilot study, six core sites used the Maestro for their MEA studies with two different cell types and eight compounds with known mechanism. The Maestro and accompanying software tools demonstrated high reliability across replicates, sites, and cell types, while accurately detecting phenotypic changes in depolarization (spike amplitude), repolarization (field potential duration), and arrhythmias.
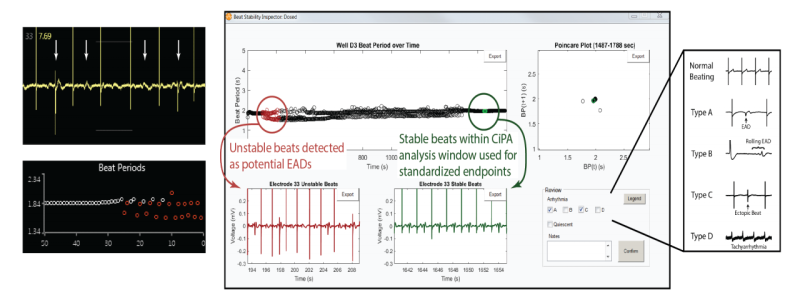
Automated CiPA protocol with arrhythmia detection
Axion‘s AxIS software enables real-time visualization of EADs, while the CiPA Analysis Tool provides automated detection of potential arrhythmias with a user interface for inspection and classification. The completed analysis can be readily exported as “publication-ready” dose response figures or in the official CiPA reporting format.