A wound healing assay, or scratch assay, measures the migration of cells across a scratch-induced gap in vitro. This type of assay can be used to monitor cell proliferation and cell migration, often in wound healing studies or in oncology research assessing the mechanisms of cancer metastasis. Continuous, label-free monitoring using Maestro and Omni live-cell analysis platforms provides a true kinetic analysis of cell migration and eliminates the guesswork associated with endpoint-only data.
Solutions for kinetic scratch assays
-
Visualize wound closure in real time>
-
Characterize cancer cell lines>
-
Investigate the effects of therapeutic interventions>
-
Discover the benefits of Axion's kinetic scratch assay>
The Omni imaging platform and Scratch Assay Software Module offer an integrated solution to monitor your cell migration in real time on any cell culture plate.
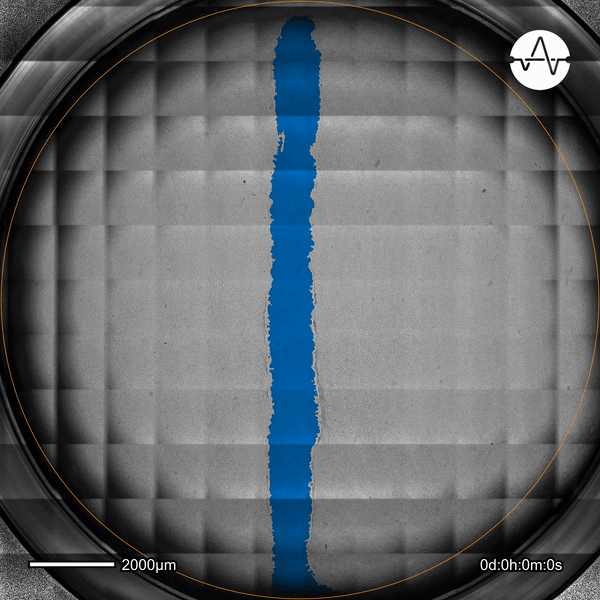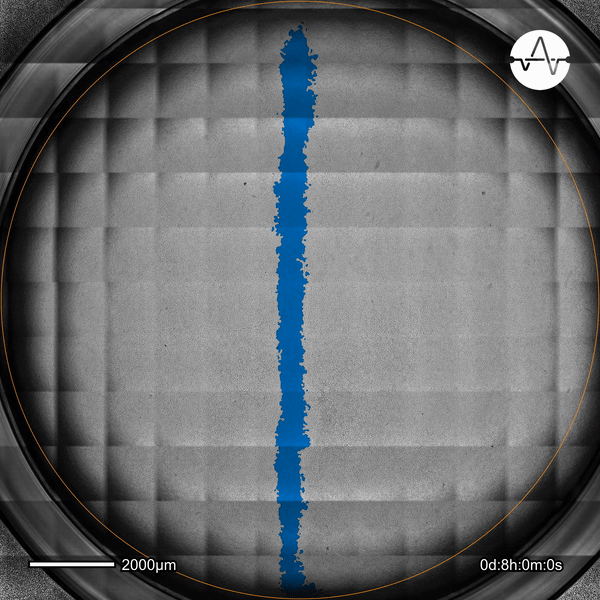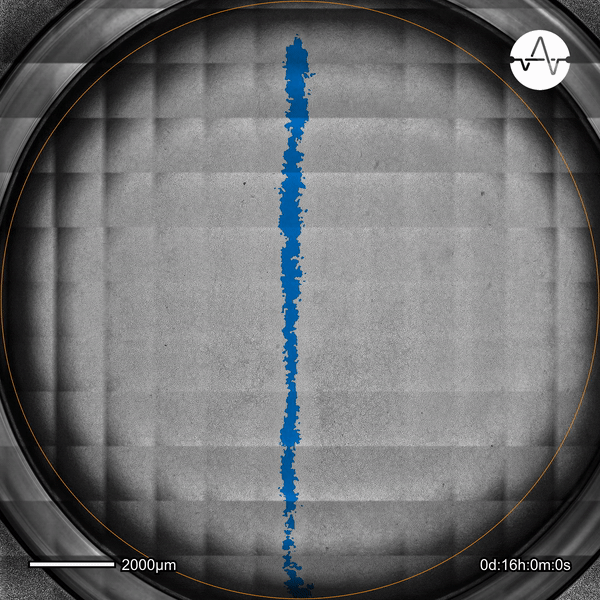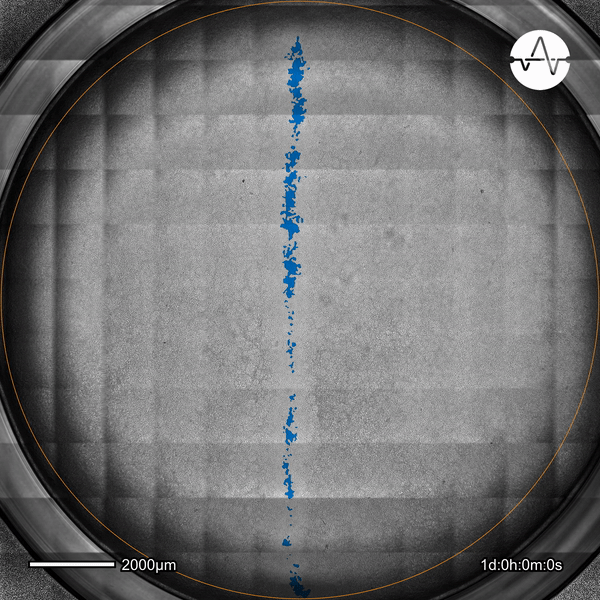
The ability of cancer cells to migrate represents a hallmark event of cancer metastasis. In this example, the Omni live-cell imager was used to examine the collective migration of glioma cells. Watch as the intuitive software automatically selects the cell-free ('scratch') area (highlighted in blue) and quantifies the gap closure and cell migration speed in timelapse video taken over 17 hours.
Cell migration represents a key factor in the metastatic cascade and a proxy for metastatic potential. See how the scratch assay can provide deep insights into the characterization of different cancer cell lines.
In this study, the Maestro Z impedance assay was used to characterize the migration of two breast cancer cell lines: MCF-7, a hormone-receptor positive line, and HCC1806, a triple-negative breast cancer line.
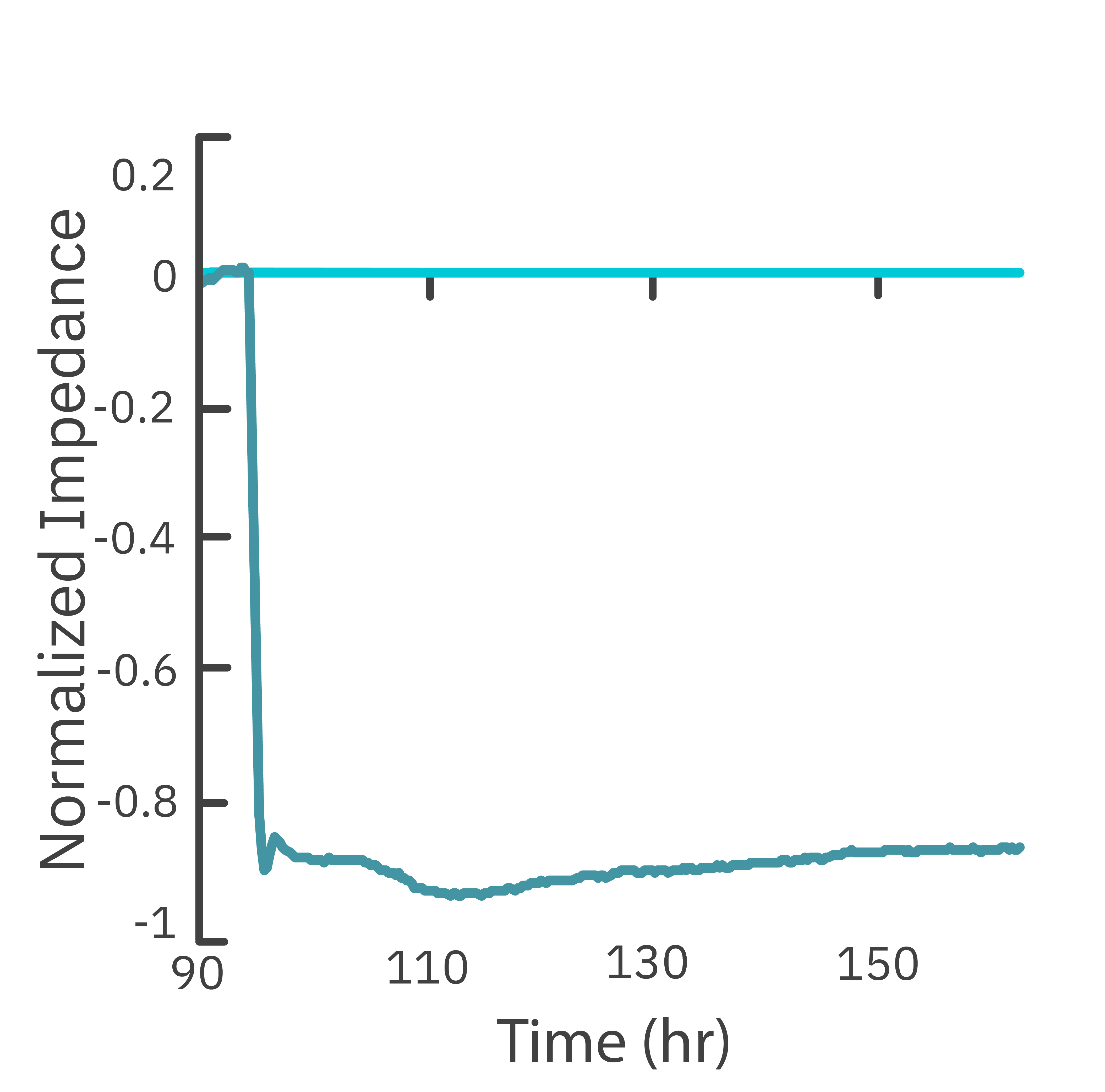
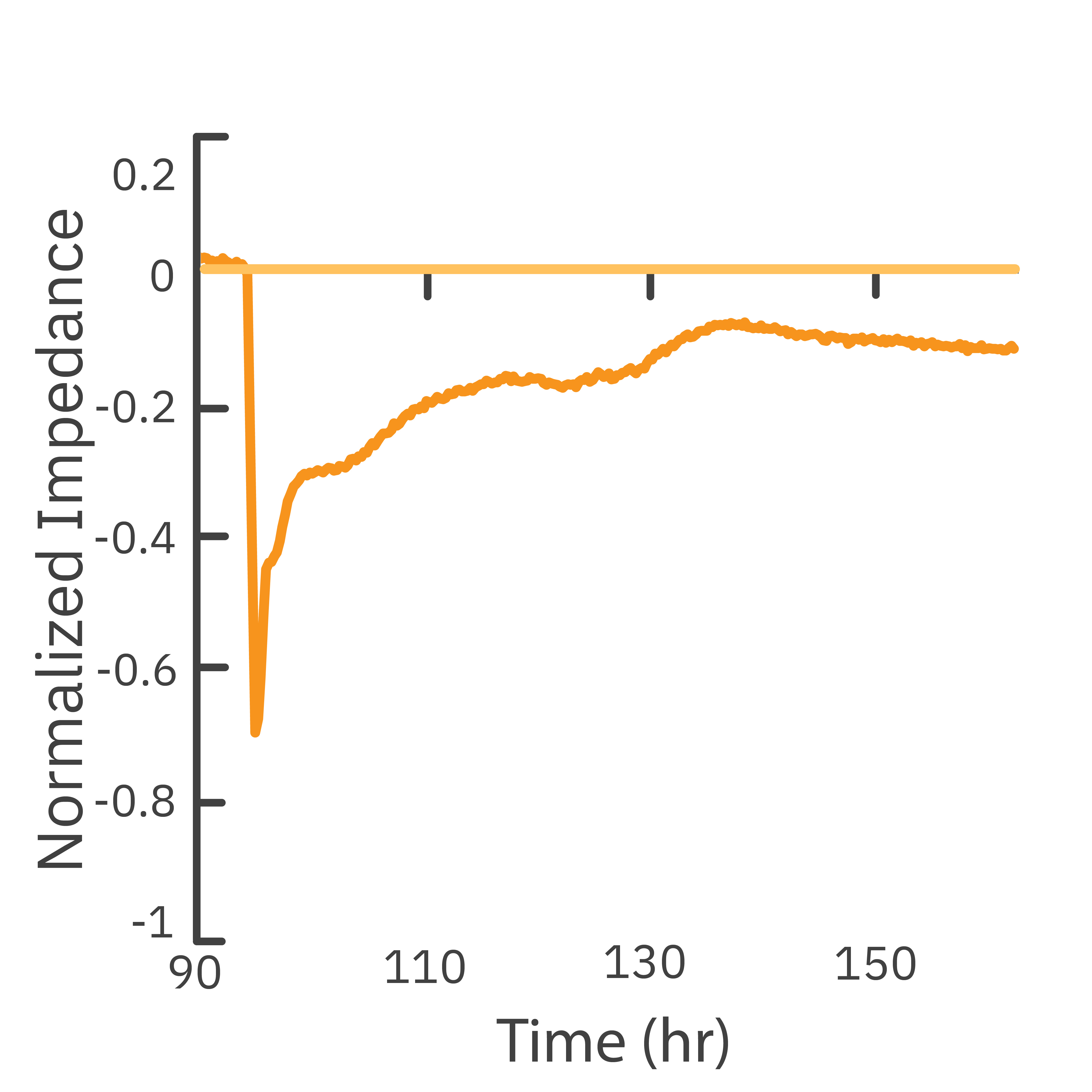
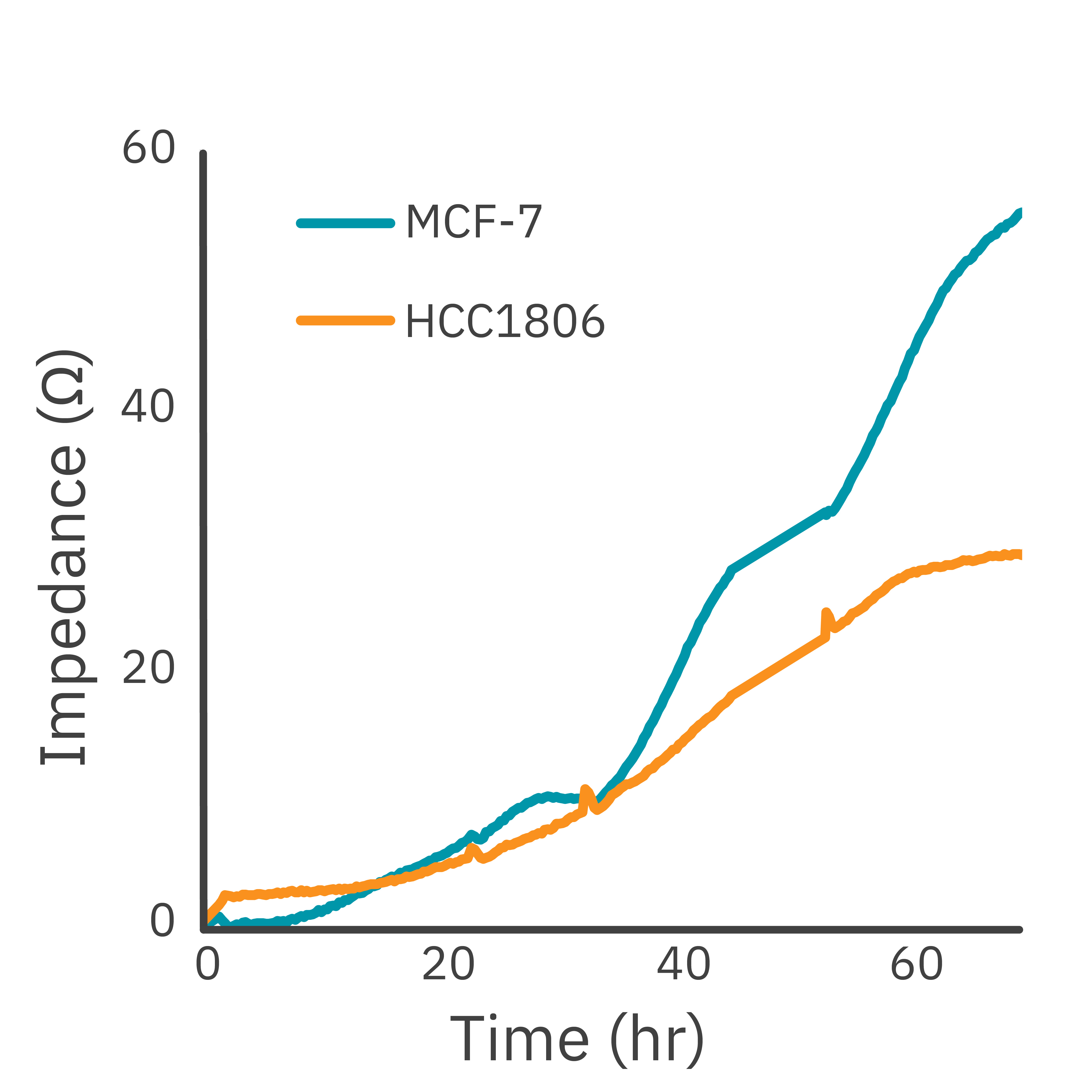
Each cell type exhibited a unique impedance profile over time during proliferation, and showed consistent decreases in impedance as a result of the scratch.
Triple negative cells HCC1806 migrated to almost fully cover the gap, whereas MCF-7 cells migrated very little over the course of 72 hours. The Maestro Z kinetic data highlighted the different cell line characterizations.
Read our application note to see the details of how the Maestro Z highlighted differences in cell lines.
The scratch assay is also an effective method to assess treatments designed to prevent cell migration.
Here, the Maestro Z clearly showed the impact of adding modified citrus pectin (MCP) to the scratch area resulted in reducing metastasis of MCF-7 and triple negative HCC1806 cells.
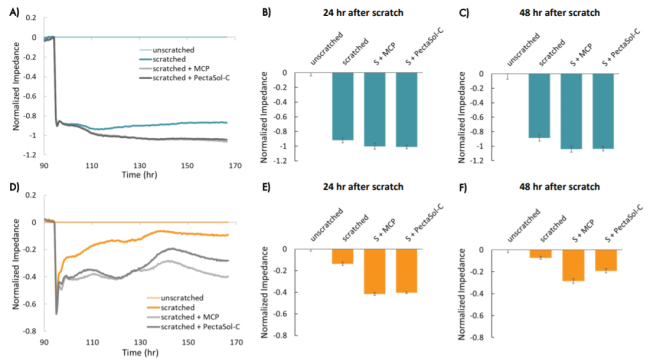
Read our app note to learn how the Maestro Z highlighted the differences in treatments on cell migration.
Axion's imaging and impedance platforms deliver live-cell kinetic data, offering several advantages compared to measurements taken at single time points.
>> Continuous monitoring: get reliable information about your dynamic cellular population response and adjust your experiments based on real-time observations.
>> Automated assessments: say good-bye to labor-intensive endpoint assays with continuous, hands-free monitoring.
>> Non-invasive or label-free assays: assess your cells without the use of labels, resulting in reproducible quantification of cell migration.
>> Maintain physiological conditions: record straight from your incubator with Omni or get built-in environmental controls with our Maestro Z benchtop device.
Scratch Assay Resources
>> Application Note
The effect of blebbistatin and cytochalasin-D on the migration capacity of mouse embryonic fibroblasts
>> Application Note
Monitoring migration of breast cancer cells using Maestro Z real-time impedance assay
>> Coffee Break Webinar:
Plant-derived pectin as a breast cancer therapy
Scratch Assay FAQ
>> What is the time point for a scratch assay?
Multiple time points are analyzed to track the progress of the cells across the scratch area. Some common time points are:
- 0 hours - immediately post scratch.
- 6, 12, 24 hours - early time points to help determine the onset of migration.
- 48 hours - intermediate time to see progress of scratch.
- 72 hours - later time point to record the near completion or completion of the migration.
The need to measure for multiple time points during this dynamic process highlights the importance of continuous cellular monitoring with kinetic assays on the Omni imager or Maestro Z impedance platforms.
>> How do you quantify a scratch assay?
The quantification is the measurement of the extent of wound closure or cell migration over time. This can be done through manual measurement with images captured at different time points, or with real-time systems such as impedance or automated image quantification systems.
>> Can scratch assays be used for drug screening?
Yes, scratch assays can be used to evaluate the effects of drugs on cell migration. These results provide insights into potential therapeutic interventions.
For Research Use Only. Not for use in diagnostic procedures.