Impedance
Impedance
Impedance: For real-time cell analysis
Impedance-based cell analysis is a well-established technique for monitoring the presence, morphology, and behavior of cells in culture. Impedance describes the obstruction to alternating current flow. To measure impedance, small electrical currents are delivered to electrodes embedded in a cell culture substrate. The opposition to current flow from one electrode to another defines the impedance of the cell-electrode interface. When cells are present and attached to the substrate, they block these electrical currents and are detected as an increase in impedance.
Impedance is sensitive to many aspects of cell behavior: attachment, spreading, shape, cell-cell connections (e.g. tight junctions), and death. Even small transient changes, such as swelling or signaling, are detectable by impedance. Because impedance is noninvasive and label free, the dynamics of these changes can be monitored in real time over minutes, hours, or even days without disturbing the biology.
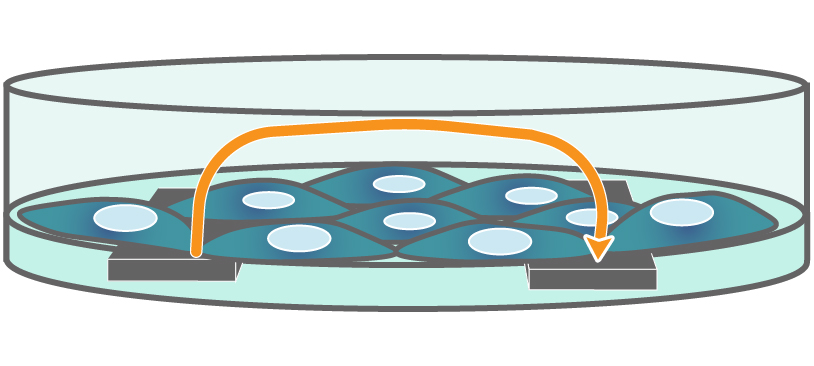
Interdigitated electrodes embedded in the cell culture substrate at the bottom of each well detect small changes in the impedance of current flow caused by cell presence, attachment, and behavior.
In the example below, the electrodes are initially uncovered before cells are added. The electrical current passes easily and the impedance is low. When cells begin to attach and cover the electrodes, less electrical current passes and the impedance is high. After dosing with a cytotoxic agent, cells die or detach, and the impedance decreases back towards baseline.
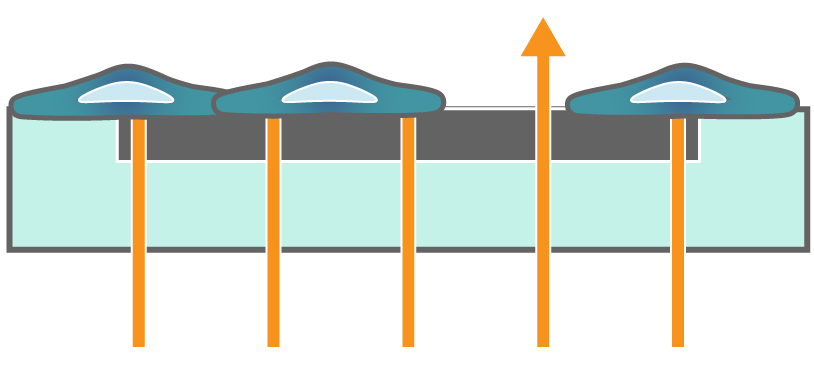
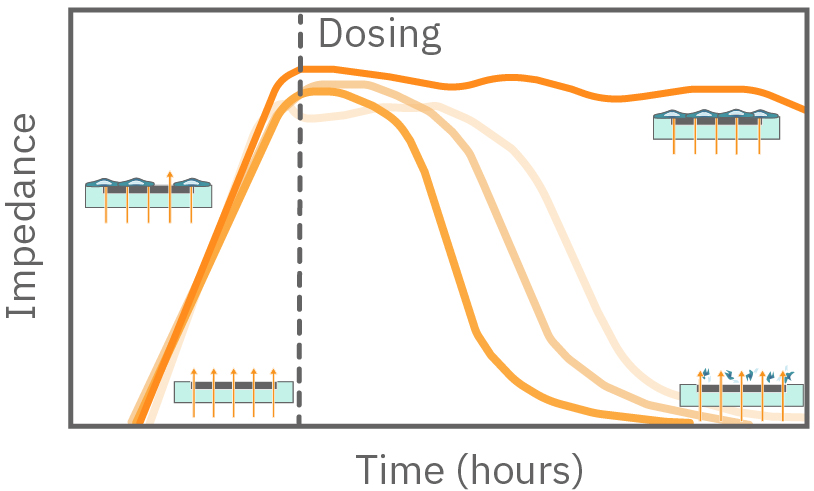
Impedance measures how much electrical signal (orange arrows) is blocked by the cell-electrode interface. Impedance increases as cells cover the electrode and decreases back to baseline due to cell death.
Continuous cell monitoring
Many cell-based assays are endpoint assays, limited to a single snapshot in time. Repeating these assays at multiple time points can be labor intensive, time consuming, and costly. Key time points can be easily missed. Impedance-based cell analysis is nondestructive and label free, meaning that cellular dynamics can be monitored continuously.
The impedance assay can be used to characterize dynamic cell profiles, revealing how cells grow, attach, and interact over time. Each cell type exhibits a different cell profile, or “fingerprint”, of dynamic cell behavior. These profiles are sensitive to cell type, density, purity, and environmental factors. In this example, the Maestro Z impedance assay readily distinguished cell profiles across different cell densities and cell types.
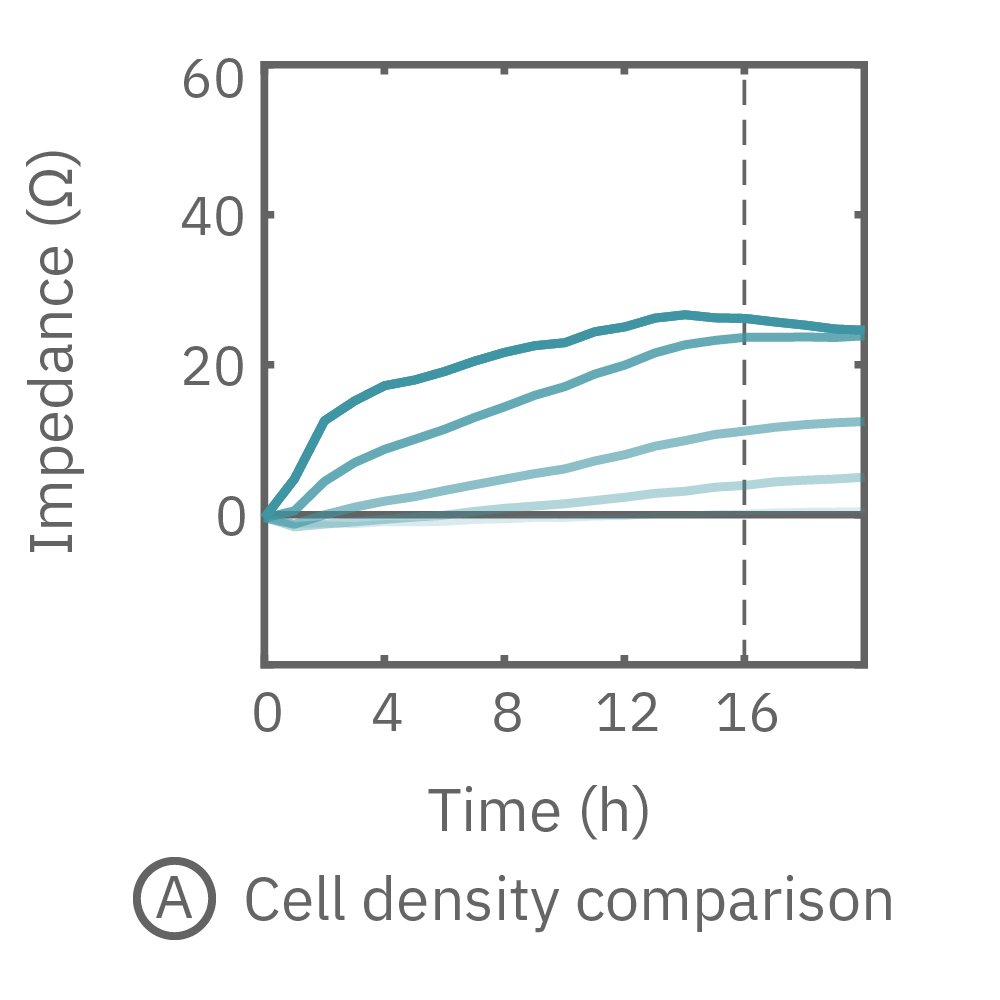
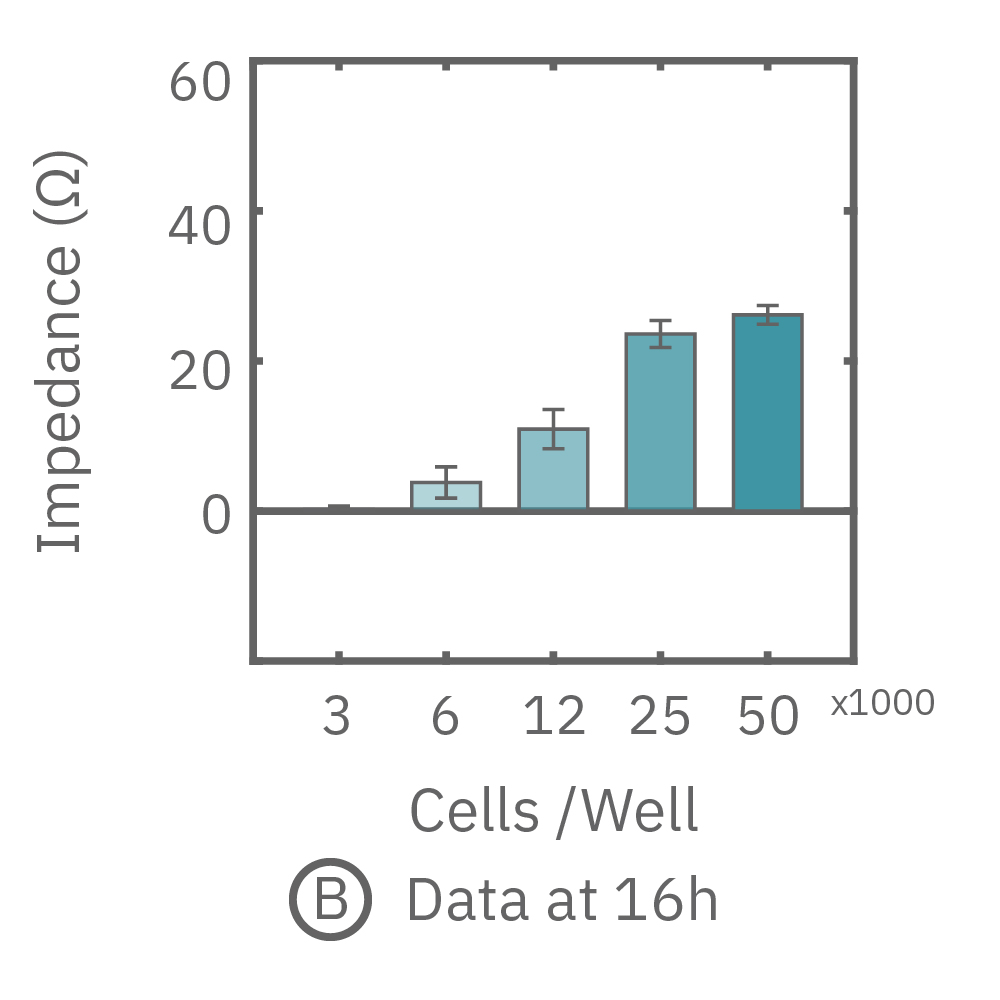
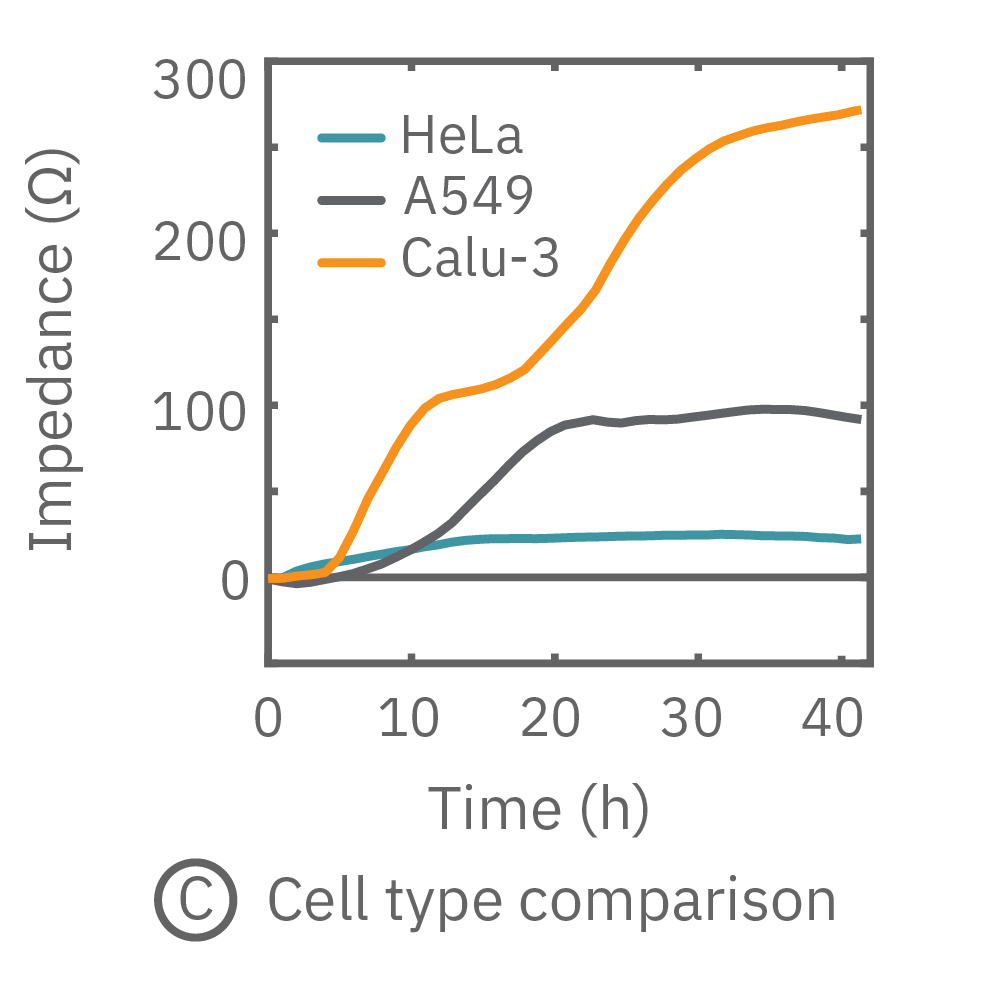
(A, B) HeLa cells were seeded on a CytoView-Z plate at varying densities and the impedance was continuously monitored by the Maestro Z. Impedance scaled proportionally with cell density and readily distinguished different densities of the same cell type. (C) Maestro monitored the growth of three cell types, HeLa, A549, and Calu-3, and readily distinguishes their distinct cell profiles over time.
The Maestro Z impedance assay can also be used to capture the kinetics of cell responses to drugs or immune cell therapies. The kinetics, which cannot be captured by endpoint assays, often provide key insights into the efficacy of novel therapies. In the example below, the Maestro Z impedance assay was used to quantify the kinetics of cytotoxicity of chemotherapy agents.
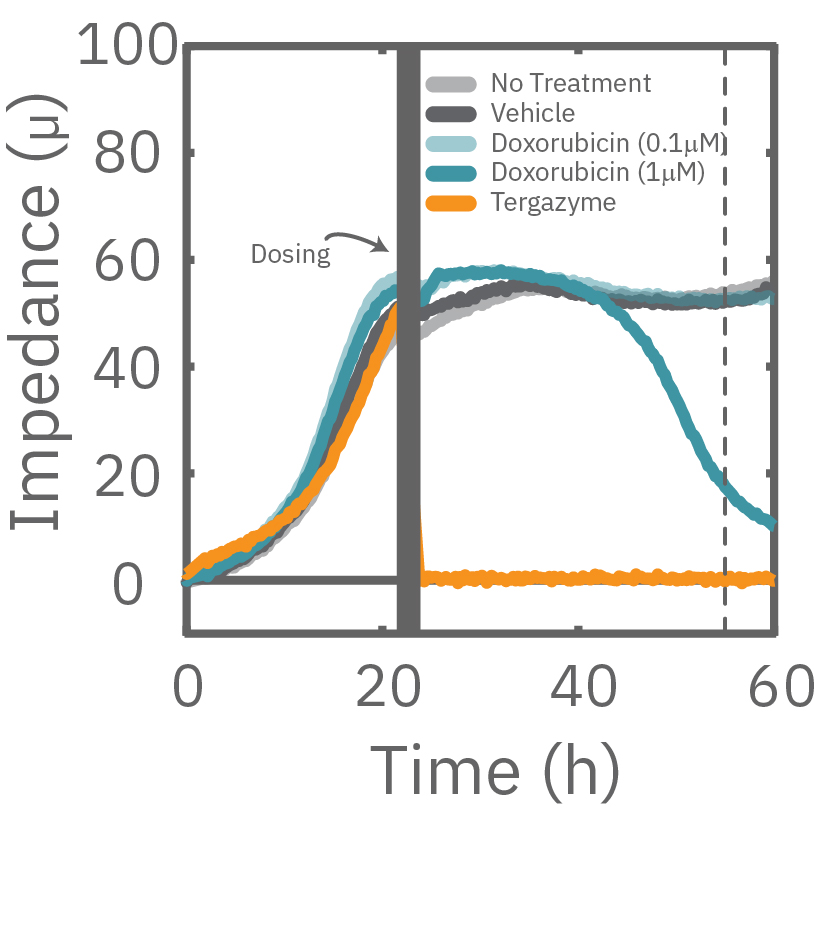
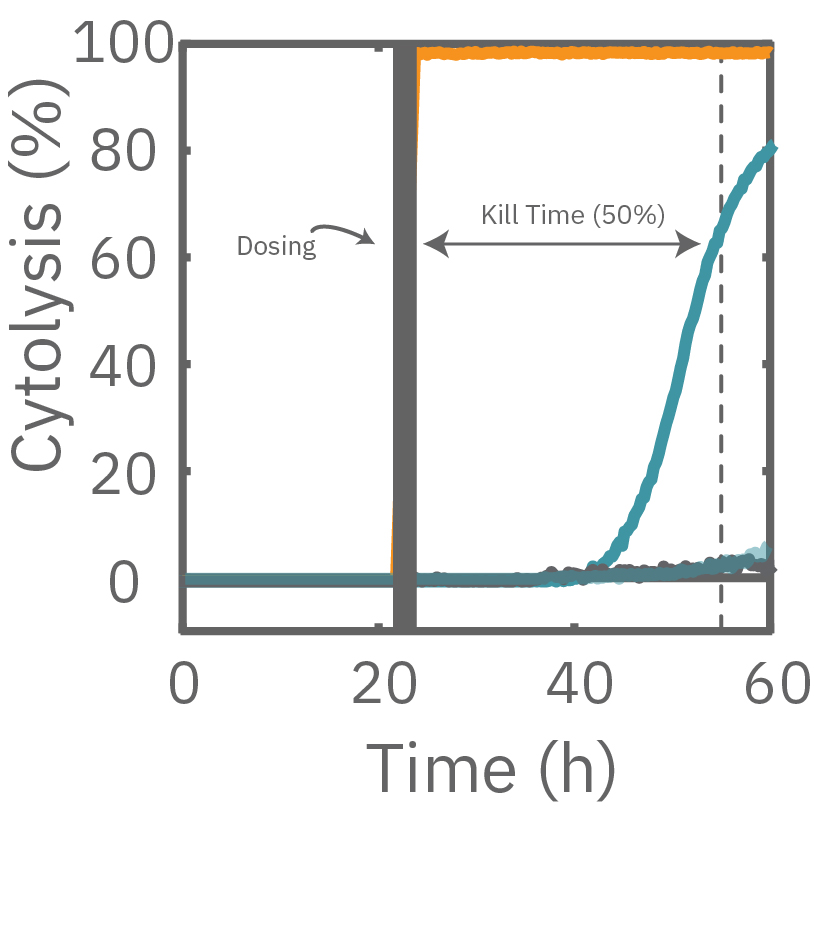
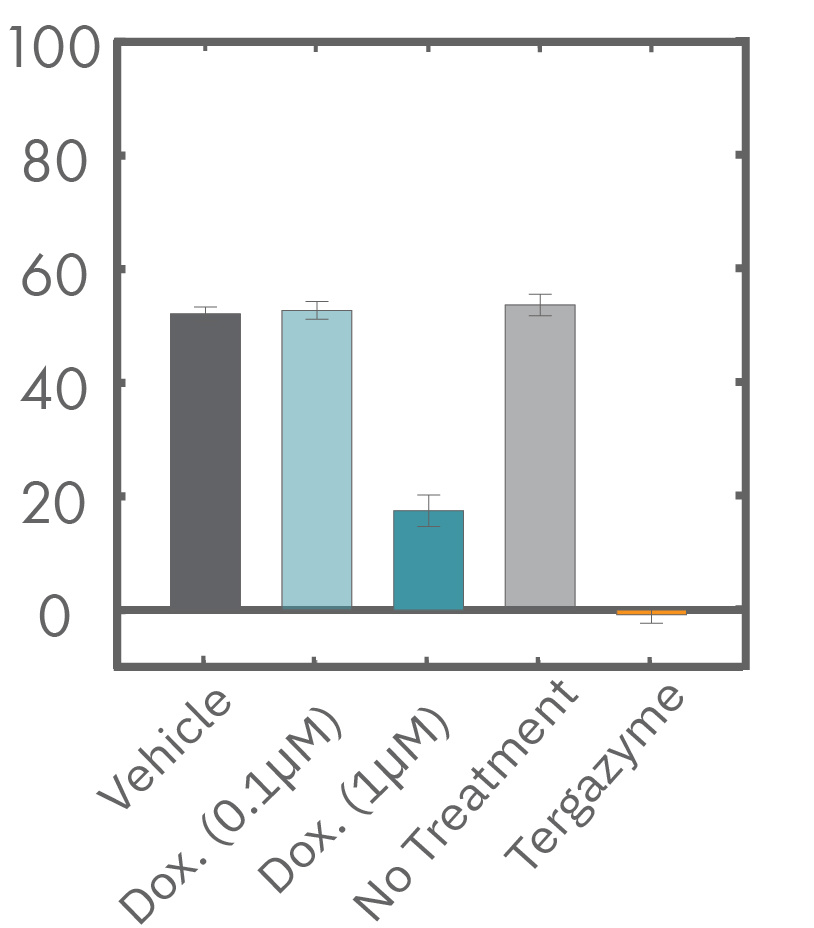
A549 cells were dosed with dox, vehicle (DMSO), or tergazyme. Wells dosed with tergazyme showed an immediate decrease in impedance, reflecting complete cell death. Higher doses of dox resulted in a slower decrease in impedance and cell death. Cells dosed with 1 μM dox reached 50% cytolysis at 31 hrs.
Different frequencies reveal cell properties
Impedance varies with frequency, such that different frequencies reveal different aspects of cell biology. The small currents used to measure impedance will always take the path of least resistance. At low frequencies, such as 1 kHz, the impedance of the cell membrane is relatively high, forcing the current to flow under and between the cells. Low frequencies provide details about barrier integrity, the presence of gap junctions, and transepithelial or transendothelial resistance (TEER).
At high frequencies, such as 41.5 kHz, the impedance (and capacitive reactance) of the cell membrane is relatively low. Thus, most of the current couples capacitively through the cell membranes, providing information about the cell layer such as confluency and coverage.
In other words, low frequencies are sensitive to “what” cells are there, whereas high frequencies are sensitive to “how many” cells are there. The Maestro Z impedance assay uses multiple frequencies to provide the most information about the cells, simultaneously, continuously, and in real time.
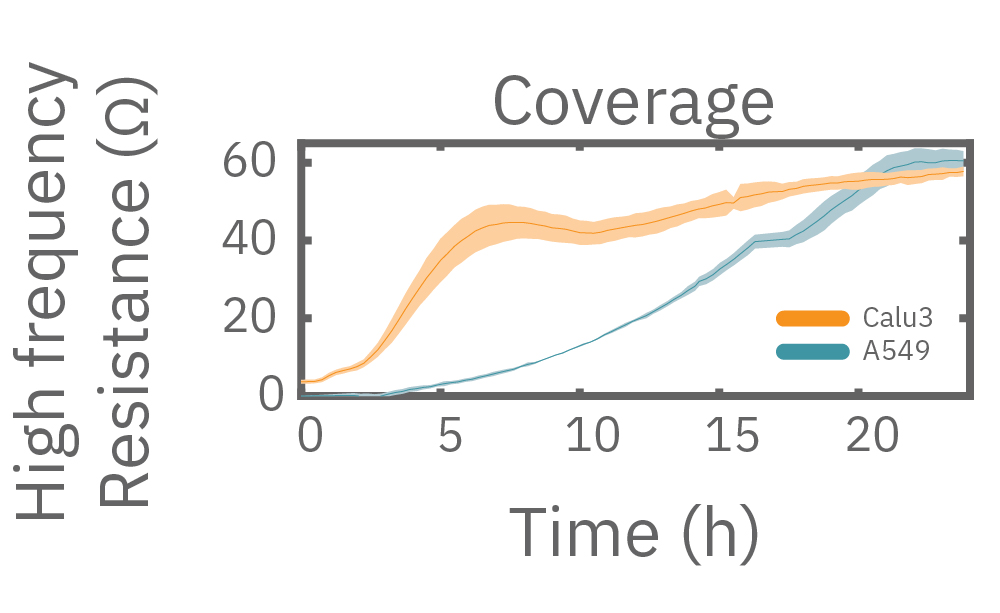
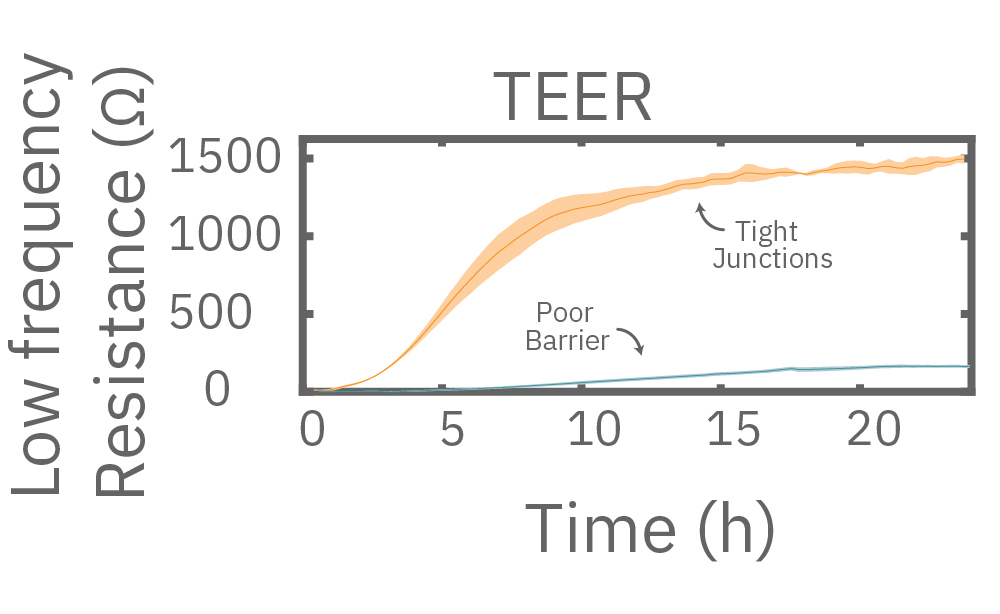
Multiple frequencies were used to simultaneously and continuously monitor the coverage and barrier function (TEER) of Calu-3 and A549 cells. Coverage, measured as resistance at 41.5 kHz, increases over time for both cell types. TEER, measured at 1 kHz, reveals that only Calu-3 cells form a strong barrier, as they express tight junctions to block flow between neighboring cells.