Sections included in this whitepaper:
Why measure neural activity?
In vitro neural models are a proven powerful strategy for the study of neural function and complex disorders of the human nervous system. Can the complexity of your neural cell models be revealed with only cell imaging, gene expression analysis, or Western blots? The neurons may have the proper characteristics, but how do they function in a network? Using Maestro microelectrode array (MEA) technology, any scientist can now quickly and easily measure electrical network behavior in live cells at high throughput.
What is MEA?
Axion’s microelectrode array (MEA) plates have a grid of tightly spaced electrodes embedded in the culture surface of each well. Electrically active cells, such as neurons, can be cultured over the electrodes. As the cultures mature over time, neurons form cohesive networks and develop functional network activity. The spontaneous firing of neurons is captured from each electrode on a microsecond timescale providing both temporally and spatially precise data. Complement your assay with evoked metrics from electrical or optical stimulation.
Neural network recordings?
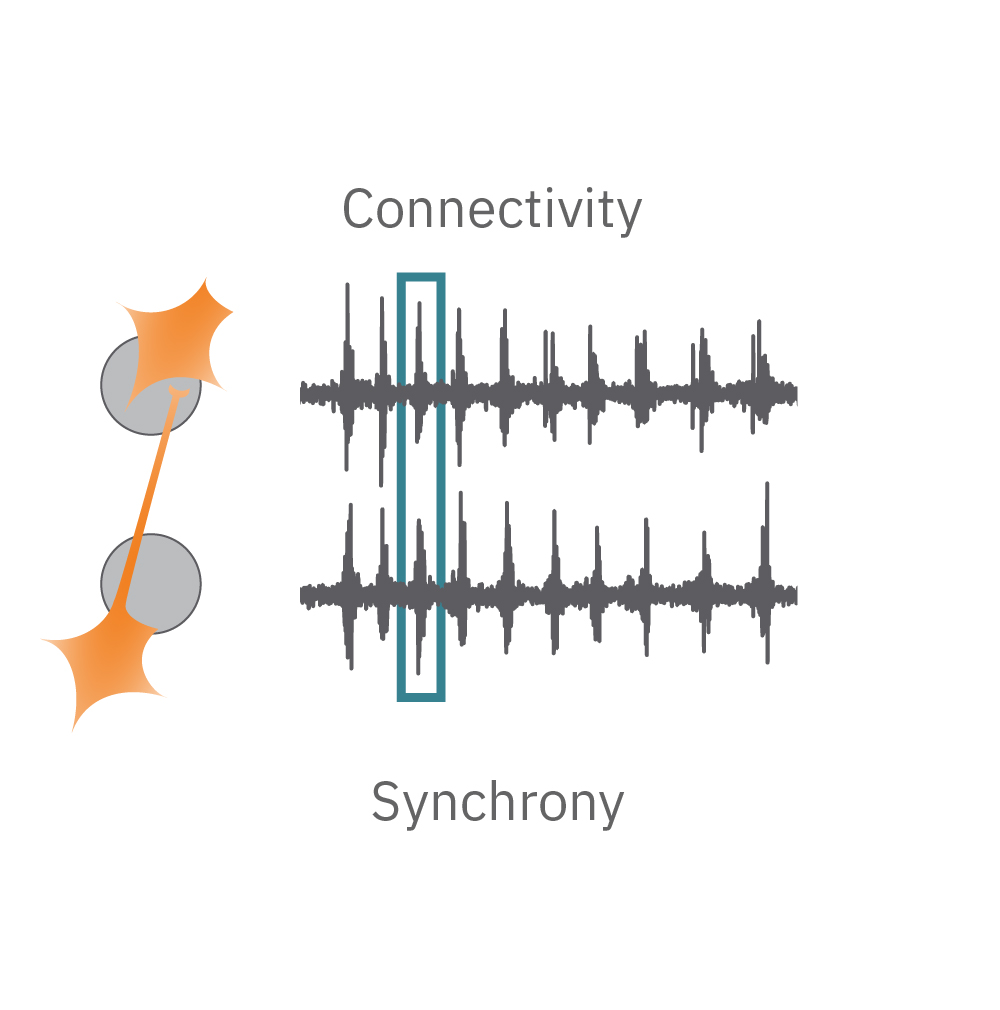
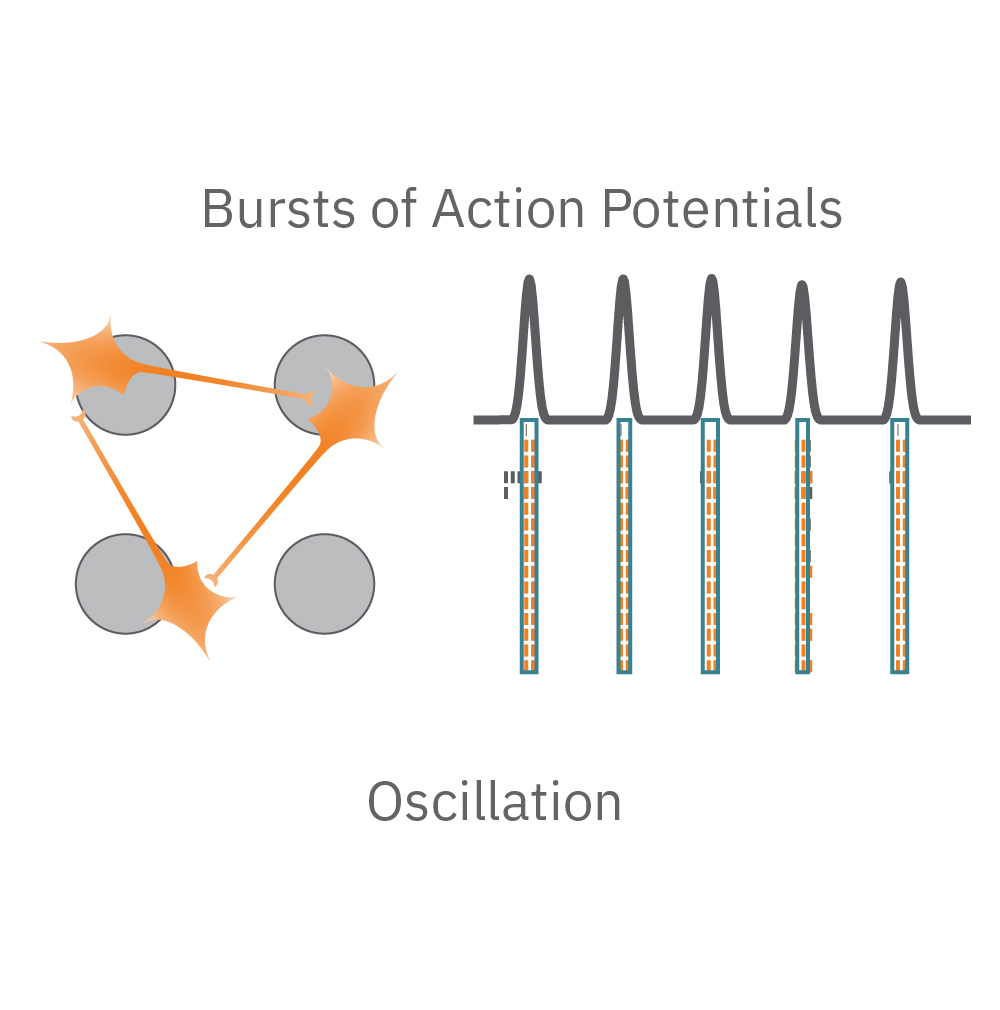
Electrical activity is captured from neurons (orange) cultured over electrodes (gray circle). The Maestro MEA system detects key parameters of neural network function, including activity, synchrony, and oscillation.
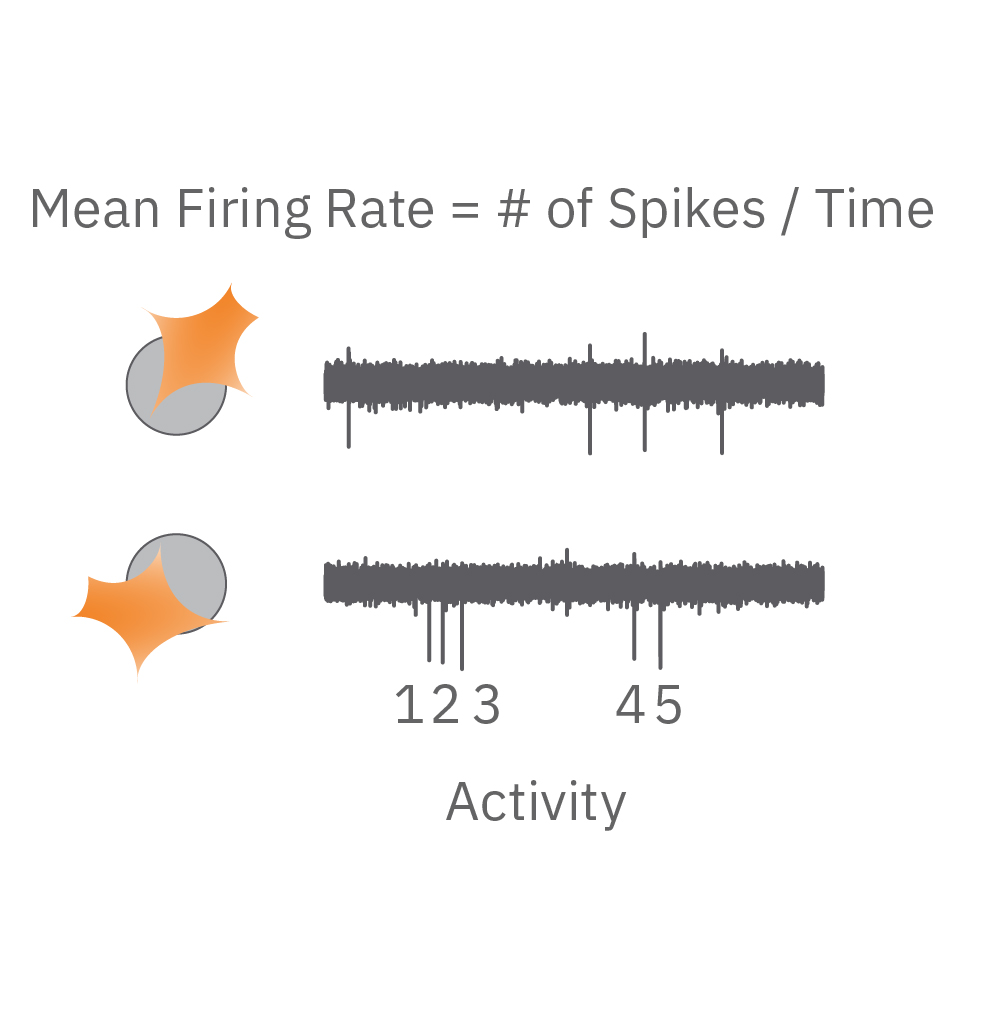
Activity – are the neurons functional? Action potentials are the defining feature of neuron function. High values indicate the neurons are firing action potentials frequently. Low values indicate the neurons may have impaired electrophysiological function.
Synchrony – are the synapses functional? Synapses are functional connections between neurons, such that an action potential from one neuron affects the likelihood of an action potential from another neuron. Synchrony reflects the strength of synaptic connections, and thus how likely neurons are to generate action potentials simultaneously on millisecond time scales.
Oscillation – is the network functional? Neural oscillations, defined by alternating periods of high and low activity, are a hallmark of functional networks with excitatory and inhibitory neurons. Oscillation is a measure of how the spikes from all of the neurons in a well are organized in time.
Fragile X
What is the Fragile X disease phenotype?
Fragile X syndrome is the most prevalent genetic form of intellectual disability with an incidence of one in 4,000. Patients with Fragile X syndrome display a broad spectrum of autistic phenotypes that range from mild to severe intellectual, social, and reasoning deficits. This disorder stems from the loss of the Fragile X mental retardation protein, FMRP, an RNA binding protein which is essential for synaptic function. Currently, there is no cure or treatment for Fragile X syndrome due in part to the complexity in the Fragile X syndrome neuronal circuitry. In the recent Cell paper, Dr. John Graef (Fulcrum Therapeutics) used the Maestro along with CRISPR gene-editing and patient-derived cells to recapitulate the Fragile X syndrome phenotype in a dish. Moreover, this approach has enabled an estimate of the level of FMRP protein expression required to correct the observed Fragile X syndrome phenotype.

How Maestro MEA assay can help?
In an iPSC-derived model of Fragile X syndrome, the cultures were first characterized by a significant reduction in FMR1 and FMRP expression, relative to an isogenic control. The corresponding network electrophysiology phenotype featured an increase in excitability (weighted mean firing rate) at 21+ days in culture. Through a variety of different genetic techniques, researchers at Fulcrum Therapeutics were able to design Maestro assays to directly link the increased network activity to FMRP expression in the network.
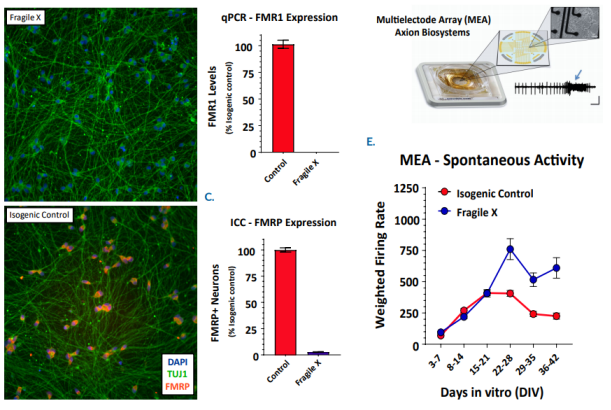
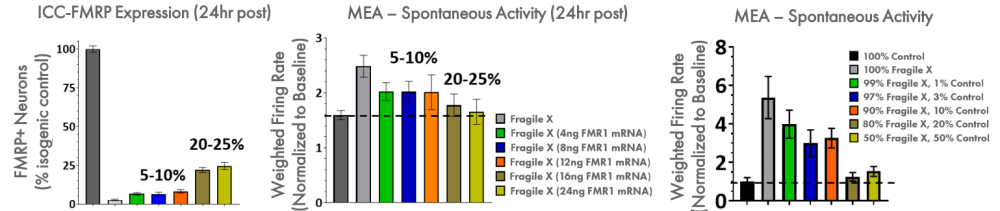
FMRP expression was re-introduced to the Fragile X model through addition of FMR1 mRNA. With increasing addition of FMR1 mRNA, the FMRP expression increased, as expected, and the spontaneous network activity decreased to levels matching the control cultures. Only 5-10% FMRP+ neurons following transient transfection of FMR1 mRNA leads to partial reduction of hyperexcitability, whereas >20% fully normalizes activity. In addition, the proportion of Fragile X neurons co-cultured with control neurons was titrated to determine the number of control neurons, and thus FMRP expression, required for the control phenotype. These data show that 20% of the neurons need to be expressing high levels of FMRP to normalize the hyperexcitability observed in Fragile X neurons.
Chronic Pain
What is the "Man on Fire" disease phenotype?
“Man on Fire” syndrome, also known as Inherited Erythromelalgia (IEM), is a chronic pain syndrome characterized by burning pain in the hands and feet. The chronic pain of most patients with IEM cannot be relieved by common pain killers making this disease a major unmet medical need. Precision Medicine is an approach that tailors the prescribed medical treatment to the individual’s genetic makeup. Dr. Yang Yang (Purdue University) is investigating the treatment of IEM using a pharmacogenomic approach. The drug responsiveness of different genetic mutations associated with IEM were probed in an in vitro Maestro MEA assay, predicting effective treatment for these IEM patients in the clinic. This precision medicine approach, guided by genomic analysis and functional profiling, provides a promising new way to extinguish the fire of the burning man.

How Maestro MEA assay can help?
Most patients with IEM and Nav1.7 mutations do not respond to any available pain medications. However, through trial and error, it has been shown that patients with a V400M mutation respond to Carbemazapine (CBZ). Structural modeling of Nav1.7 reveals another disease-causing mutation, S241T, in close proximity to V400M. In this study, researchers found that neurons expressing the Nav1.7-S241T mutation fire significantly more action potentials than WT neurons, and this effect is exacerbated with increasing temperatures. When these neurons were treated with CBZ, the firing frequency was greatly reduced, suggesting CBZ as a potential treatment for IEM patients with S241T mutations.
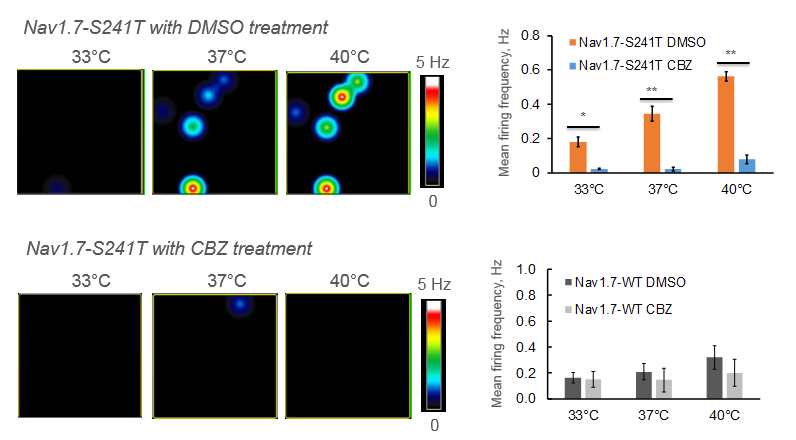
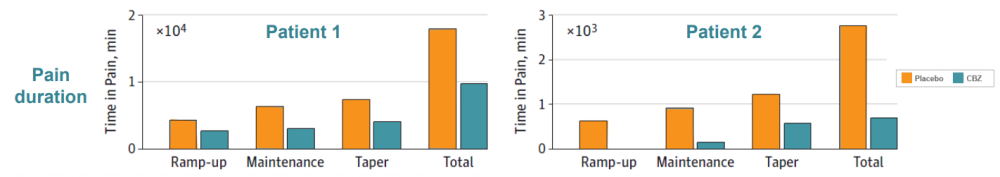
Two IEM patients (mother and son) were identified carrying the S241T mutation. Both subjects had the typical IEM pain phenotype, reporting severe burning pain in the feet, triggered by mild warmth. These patients were given CBZ in a randomized, placebo-controlled, double-blind, cross over clinical study. Pain duration, episode duration, and awakenings because of pain were reduced in CBZ treatment compared to the placebo. Moreover, functional MRI data showed that CBZ was able to switch their brain activity from a chronic pain state to an acute pain state (data not shown), further demonstrating that CBZ has a beneficial effect on patients carrying the S241T mutation.
Autism
What is the Autism disease phenotype?
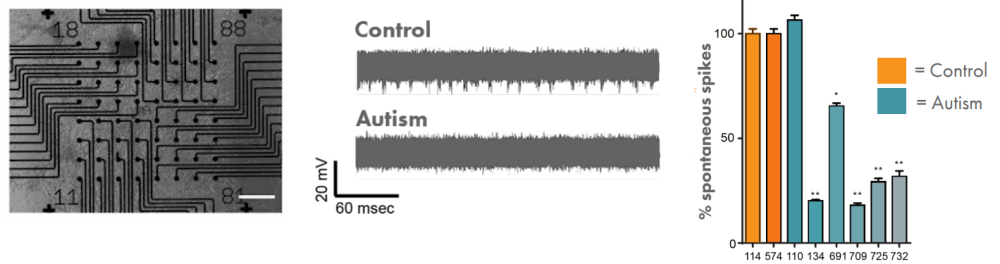
Autism, also known as autism spectrum disorder, is a range of conditions classified as neurodevelopmental disorders. Individuals diagnosed with autism show challenges with social skills, repetitive behaviors, speech and nonverbal communication. Autism is estimated to affect about 1% of people, or 62.2 million globally. While specific causes of autism have yet to be found, there is a strong genetic basis to the disorder. The genetics of autism are complex meaning better methods are required to help understand the genetic risk factors that underlie autism. Dr. Michael Nestor (The Hussman Institute for Autism) is studying the spontaneous firing activity of patient-derived iPSC neurons in a Maestro MEA assay to help to build a model of autism.

How Maestro MEA assay can help?
Human stem cell-derived cortical neurons from individuals with autism were used to model aspects of the condition. Spontaneous spike activity was decreased in individuals with autism relative to control.
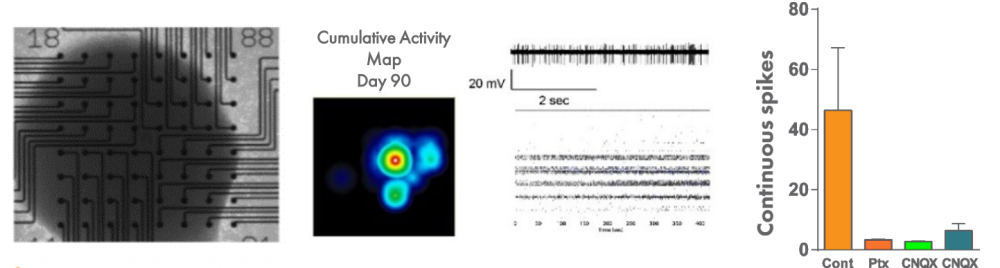
In order to understand the dynamics of networks in a more complex system of autism, 3-D organoid-like cortical structures, called serum-free embryoid bodies (SFEBs), can be recorded using the Maestro MEA system. Spikes were recorded from SFEBs up to and beyond day 90, and spontaneous spiking was disrupted by applying drugs that affect the balance of excitation and inhibition.
Neuromuscular disorders
What is the neuromuscular disorder phenotype?
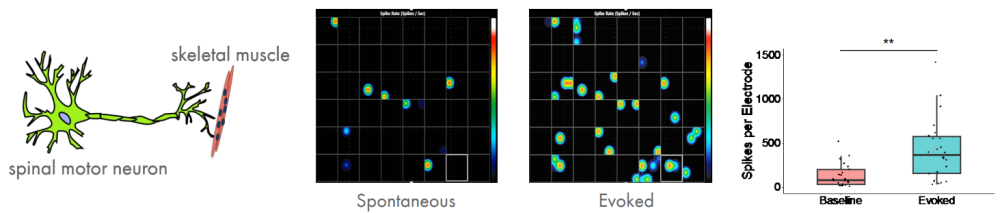
The neuromuscular junction is the synapse formed between a motor neuron and a muscle fiber. Diseases that impair the functioning of muscles, either directly or indirectly via motor neurons, are referred to as neuromuscular disorders. Neuromuscular disorders include ALS, myasthenia gravis, and the muscular dystrophies such as Duchenne's. Collectively these disorders exceed an incidence of 1 in 3,000. Although there is a strong genetic understanding of many of these disorders, the poor translatability of animal models to humans has hindered the development of treatments for these diseases. Consequently, there is a need for a model that more faithfully recapitulates the physiology of the human neuromuscular junction. Elliot Swartz (UCLA) is building a light-controlled hiPSC model of a neuromuscular junction to better understandneuromuscular disorders.

How can Maestro MEA assay help?
To establish a neuromuscular model, motor neurons and skeletal muscle were derived independently, and then recombined at a later stage, leading to functional neuromuscular connections in less than 7 days in vitro. To gain experimental control over the system, channelrhodopsin was expressed in the iPSC-derived motor neurons. Cocultures were stimulated in a 48-well MEA plate using the Lumos optical stimulation device and the resulting electrophysiological activity was recorded. Optical stimulation increases the number of spikes and bursts per electrode, as well as the network activity, as expected.
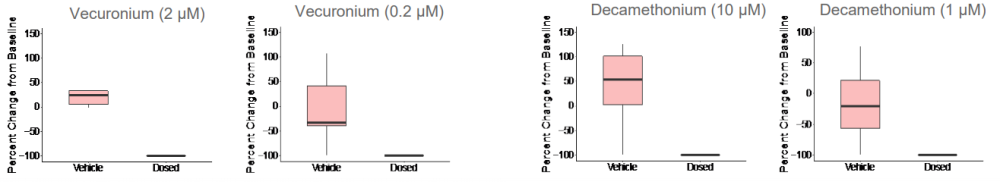
Given that both skeletal muscle and motor neurons are electrophysiologically active, neuromuscular antagonists vecuronium and decamethonium bromide were applied to the networks to block the neuromuscular junction. Evoked spike counts, or spikes that occur in a 500 ms window following light stimulation, were quantified. Both vecuronium and decamethonium completely abolished evoked activity, suggesting that recorded activity is mediated by neuromuscular transmission.
Maestro MEA systems
How does a MEA assay work?

Getting started with Maestro couldn't be easier. Culture your neurons in an Axion multiwell MEA plate [A]. Load this MEA plate into the Maestro MEA system and allow the environmental chamber to automatically equilibrate [B]. Analyze the activity of the neurons in the MEA plate label-free and in real-time with AxIS Navigator software [C]. Neural activity can be quantified with three comprehensive endpoints (activity, synchrony, and oscillation) or you can dig deeper into over 25 automatically-generated endpoints.
The Maestro Advantage
-
Measure what matters – In contrast to regularly-used indirect measures of excitability, the Maestro MEA system directly measures the action potentials. Indirect measures of excitability, such as calcium imaging, are unable to capture important but subtle changes to neural network signaling, and protein expression levels often poorly correlate with cell model performance. The Maestro MEA system tracks cell excitability in real-time enabling you to answer the question that matters: do your hiPSC-neurons fire as expected?
-
Analyze cell activity label-free – The Maestro MEA system performs noninvasive electrical measurements from the cultured neural population, circumventing the use of dyes/reporters that can perturb your cell model and confound results. Track activity over hours, weeks, and months from the same population of cells
-
Probe cell models in the same plate they were cultured in – other higher throughput platforms (e.g. automated patch clamp, flow cytometry) often require cell samples to be transferred into a single-cell suspension before testing. This is far from ideal since excitable cells exist as a functional network of inter-linked cells. In addition, the cell harvesting process requires numerous handling steps. The Maestro MEA system captures neural excitability while preserving the morphological complexity of your neural cell model.
-
It's easy – you don't have to be an electrophysiologist to use the Maestro MEA system. Just culture your neurons in an MEA plate, load your plate into the Maestro MEA system, and record your neural data. Axion's data analysis tools will do the rest, even generating the publication-ready graphs you need.




