Request Information
急速に発展する免疫療法研究では、体内の免疫系を利用して腫瘍を標的・攻撃する救命治療法が次々と生み出されています。Axion BioSystems のライブセル解析プラットフォームは、簡単操作とシンプルなワークフローで免疫細胞キリングアッセイを行うことが可能です。潜在的な候補化合物を迅速かつ効果的に評価し、意思決定のための信頼性の高いデータを得ることができます。免疫調節剤の研究から、固形および液性腫瘍に対するCAR-T細胞キリングの評価まで、免疫療法の研究開発を加速させます。
Immuno-oncology assays: Get more with live-cell analysis
免疫治療法は、多くの重要なステップを経て開発されます。それぞれのステップにおいて正しい選択をするために、適切なアッセイを行うことはとても重要です。
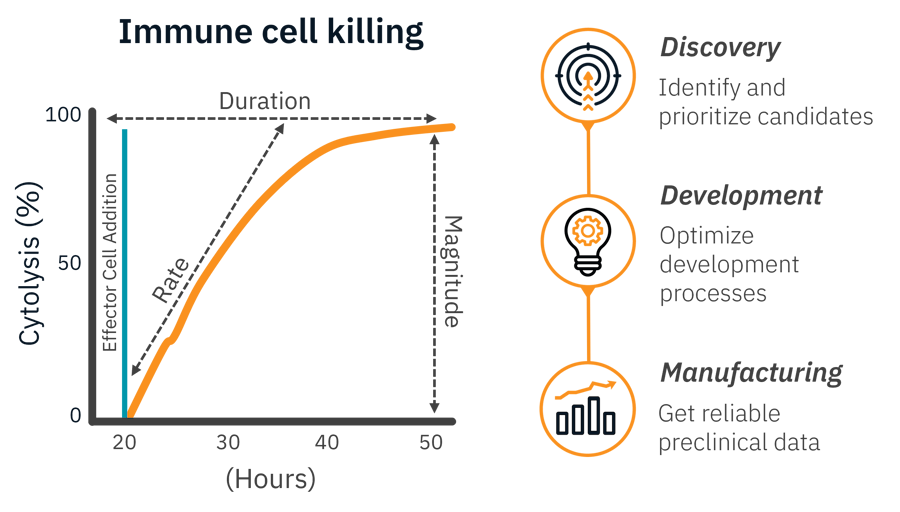
当社が提唱するライブセルアッセイでは、実験の全容を捉えることが可能です。一度の簡単なアッセイで、傷害の速度、持続時間、大きさを分析します。
候補化合物の同定と優先順位付け
- チェックポイント阻害剤のスクリーニング、CARコンストラクトの比較、免疫調節剤のテストなどにおいて、経時的な追跡が可能です。
開発プロセスの最適化
- 明確な機能データにより、形質導入メソッド、増殖、凍結保存を評価します。
信頼性の高い前臨床データを取得
- アッセイに際し試薬は不要。GMP適合システムによる、クリーンで再現性のあるポテンシーデータが取得できます。
オンコロジーアッセイ例:
CAR-T細胞キリングアッセイ
T細胞は、遺伝子操作により、腫瘍細胞上の特定の抗原を認識し攻撃します。詳細はこちら>>
NK細胞キリングアッセイ
ナチュラルキラー細胞 (NK細胞) は顆粒リンパ球であり、ウイルスや腫瘍に対する自然免疫反応に関与します。詳細はこちら>>
抗体依存性細胞傷害 (ADCC)
抗体ががん細胞に結合し、免疫細胞のキリングを増強させる免疫反応です。詳細はこちら>>
固形がん・がん細胞スフェロイド
腫瘍微小環境などの障壁により、固形癌に対する免疫療法開発は困難を伴います。がん細胞スフェロイドに対する免疫細胞キリングを検証します。詳細はこちら>>
液性腫瘍アッセイ
白血病、リンパ腫、骨髄腫などの液性腫瘍は、腫瘍免疫学において重要なターゲットです。詳細はこちら>>