TEER
TEER Assay: Barrier function on the Maestro Z
Endothelial and epithelial cells form barriers throughout the body that serve to protect and compartmentalize, regulating what gets in and out of the tissue. Endothelial cells line the inside surfaces of the body, such as the inner layer of blood vessels, while epithelial cells line the outside surfaces, such as skin and most organs. Both cell types express tight junctions, allowing them to link tightly with neighboring cells to form a selectively permeable barrier.
These important barriers can be disrupted by disease, injury, infection, or drugs. Thus, measuring barrier integrity and permeability is vital for understanding disease and predicting drug behavior.
The Maestro Z can continuously monitor barrier integrity via measurements of electrical resistance across the cell layer, a technique known as transepithelial or transendothelial electrical resistance (TEER).
How does TEER work on the Maestro Z?
The Maestro Z uses impedance technology to measure TEER, which is used to assess the barrier integrity of cells. TEER quantifies how much of an electrical signal is blocked by a cell layer when a small AC current is passed from one electrode to another. Since impedance is noninvasive and label free, barrier integrity can be continuously monitored for minutes, hours, or even days without disturbing the cellular biology.
Traditionally, TEER is measured by placing two chopstick-style electrodes on either side of a transwell insert with a confluent cell layer. Manual methods, where the electrodes are placed well-by-well, are highly labor intensive. Even automated TEER methods are typically constrained to lower throughputs, like 24-well plates. In contrast, TEER on the Maestro Z is high throughput and hands free, allowing measurements across 96 wells simultaneously.
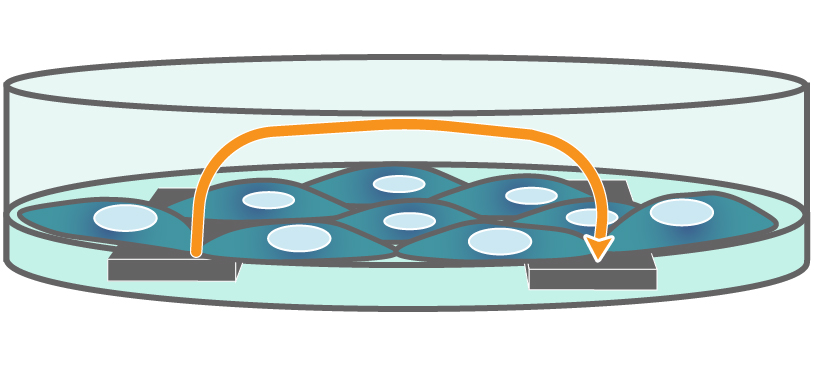
TEER on Maestro Z. Electrodes embedded in the cell culture substrate at the bottom of each well detect small changes in the impedance of current flow. Barriers, such as tight junctions between cells, resist current flow, leading to an increase in TEER measurements of barrier function.
Measure coverage and TEER in the same assay
Traditional TEER measurements require a fully confluent cell layer to accurately measure barrier function. In contrast, the Maestro Z tracks coverage and TEER simultaneously and continuously by measuring impedance at multiple frequencies.
Measuring impedance at low frequency is highly sensitive to the intercellular barrier formed by tight junctions and the paracellular barrier formed by cell membranes. On the other hand, measurements at higher frequency can be used to quantify coverage of the well bottom. In other words, low frequencies are sensitive to “what” cells are there, whereas high frequencies are sensitive to “how many” cells are there. By calculating the ratio of resistance at these two frequencies, the Impedance Module can normalize TEER to the cell coverage and give the result as barrier index.
In this example, Maestro Z resistance measurements were used to continuously and simultaneously monitor cell coverage and barrier function of two human lung epithelial cell lines. Both cell lines reach full coverage within 24 hours. However, only the Calu-3 cells, which express tight junctions, produce a significant low-frequency TEER signal, indicating a strong cellular barrier
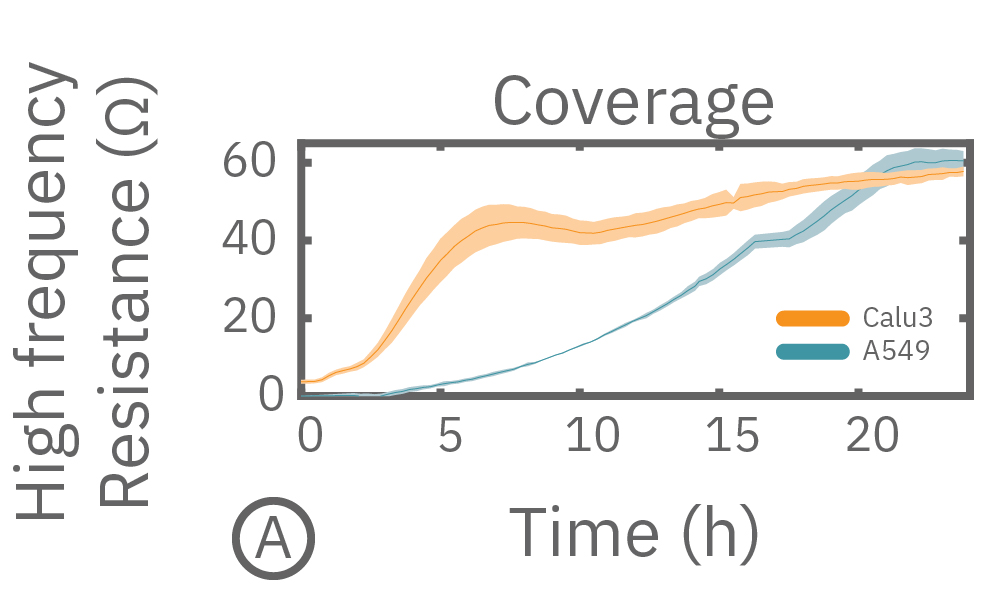
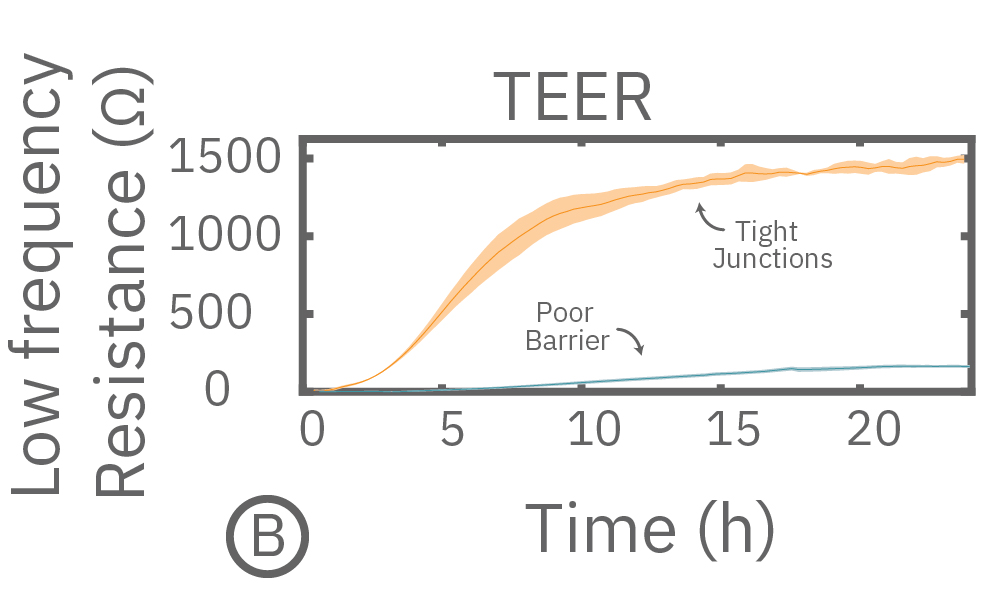
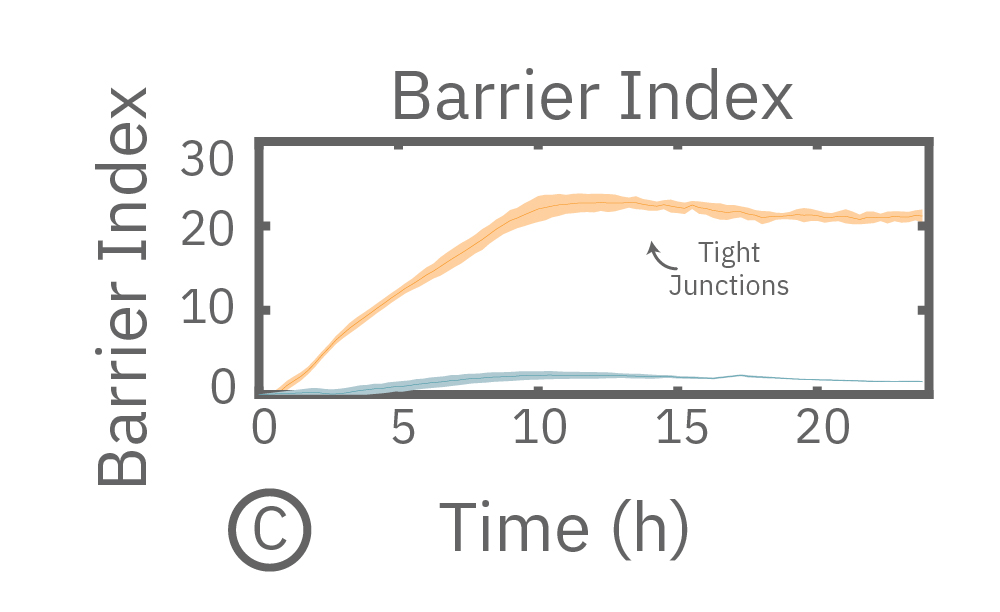
(A) Coverage, measured as resistance at 41.5 kHz, increases over time for both Calu-3 and A549 cells. (B) TEER, measured at 1 kHz, reveals that only Calu-3 cells form a strong barrier though, as they express tight junctions to block flow between neighboring cells. (C) Barrier Index both reveal that only Calu-3 cells form a strong barrier though, as they express tight junctions to block flow between neighboring cells.
Real-time detection of rapid barrier disruption
In addition to long-term monitoring, TEER on the Maestro Z is sensitive to small, transient disruptions to barrier function. Many signaling molecules in the body can alter barrier permeability. TEER on the Maestro Z can capture these disruptions in real time without being limited to a single endpoint measurement.
Here, TEER was monitored continuously for Calu-3 cells in a 96-well CytoView-Z 96-well plate on the Maestro Z. After reaching confluence, cells were dosed with cytochalasin D and ethanol. Cytochalasin D inhibits actin polymerization to increase tight junction permeability, as reflected by the rapid decrease in TEER. Ethanol acts more slowly on ZO-1 and Claudin-1, two key tight junction proteins. The rapid disruption, as well as the mechanistically distinct dynamics, are captured by TEER measurements on the Maestro Z.
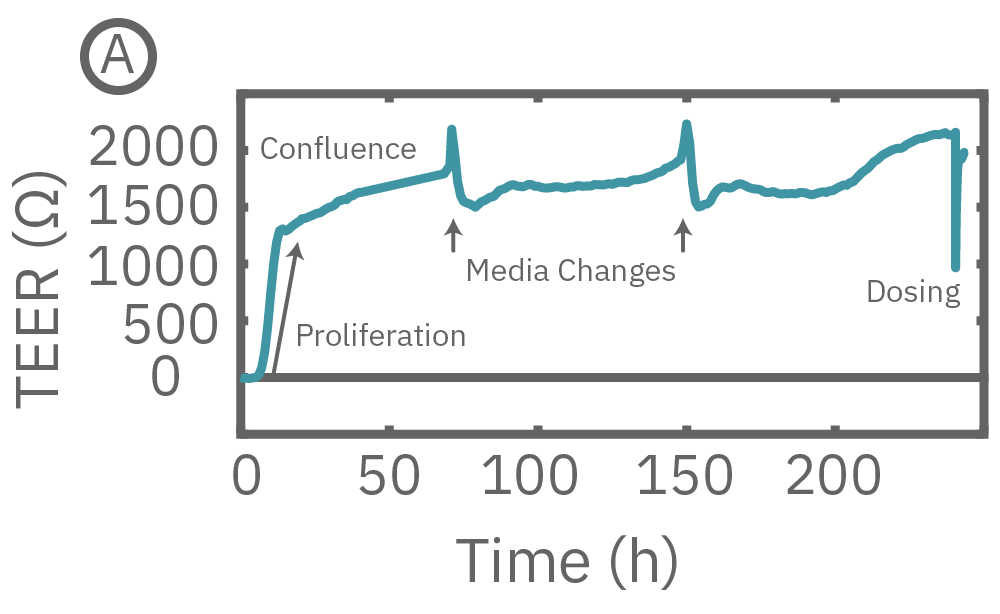
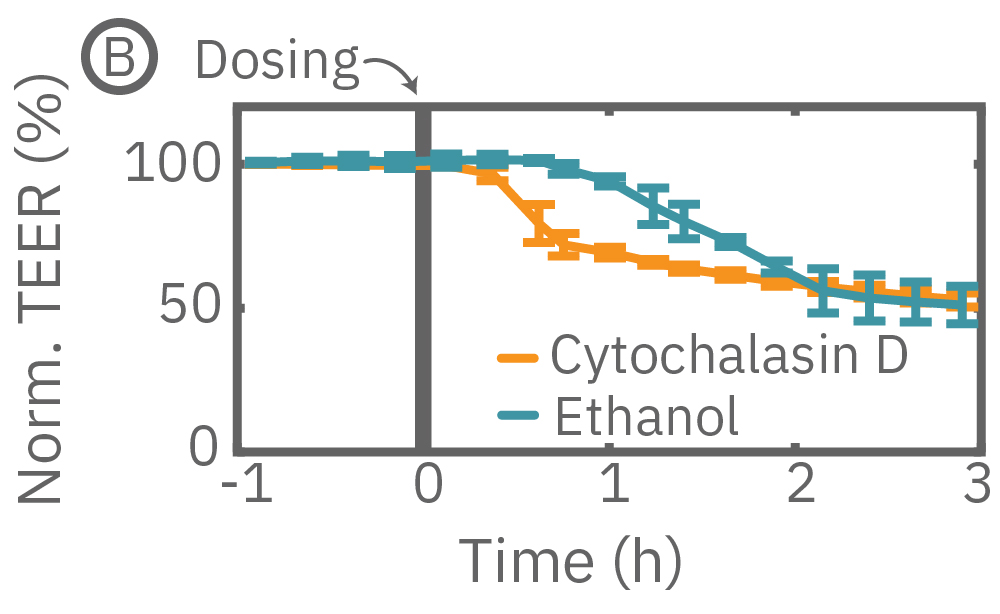
(A) TEER can be measured over the course of proliferation and barrier formation. (B) TEER is highly sensitive to transient drug-induced changes in barrier permeability, such as those induced by cytochalasin D and ethanol.