Anna-Maria Georgoudaki, Adil D. Duru, Simone Mantesso, Didem Ozkazanc, Jan Spanholtz
Glycostem Therapeutics Research Department, Oss, Netherlands
Austin Passaro, Stacie Chvatal, Konstantinos Gkatzis, Denise Sullivan
Axion Biosystems, Atlanta, GA
Key Findings:
- Real-time antibody-dependent cellular cytotoxicity can be quantified label-free with the Maestro Z.
- Trastuzumab-mediated ADCC is dependent on the effector:target ratio.
- ADCC is observed across multiple donors.
- The Maestro Z is used to investigate the mechanisms of ADCC.
Abstract
Trastuzumab promotes natural killer cell activation through antibody-dependent cellular cytotoxicity (ADCC). Understanding ADCC can help researchers develop immunotherapies with better targeting and efficacy. In this study, we examine and quantify ADCC-specific killing using the monoclonal antibody trastuzumab and peripheral blood natural killer cells on the Maestro Z. The ADCC response between donors and across multiple effector to target cell ratios is compared.
Introduction
Immunotherapy harnesses the power of the innate and adaptive immune system in various ways to seek out and attack cancer. The approach has transformed the treatment landscape for a variety of solid tumor types and hematological malignancies, but many cancer patients have limited responses to immunotherapies and efforts to improve therapeutic efficacy are ongoing. Studies have shown that variability in response to antibody-based immunotherapies such as trastuzumab, cetuximab, and rituximab may be related to the activation of effector immune cells mediating antibodydependent cellular cytotoxicity (ADCC).1
ADCC is a complex mechanism of the immune system in which monoclonal antibodies (mAbs) recognize tumor-selective antigens and bind to the surface of cancer cells. Then the immune cell, typically through the Fc receptor, recognizes and binds to the tumor-bound antibody. The immune cell then releases cytotoxic factors to induce cancer cell death. Although several cell types have demonstrated the ability to mediate ADCC, natural killer (NK) cells appear to be the most significant in immunotherapy response.2 Enhancing ADCC responses is a promising approach for improving treatment outcomes.3 To better understand interactions between immune cells and cancer cells, as well as hasten the development of mAbs engineered with improved functionality, robust in vitro assays to measure ADCC are essential. Impedance-based assays have demonstrated a superior ability to quantify this response.4
In this study, we examine the ability of trastuzumab to enhance peripheral blood natural killer (PBNK) cell-mediated killing of SKOV-3 cells. Trastuzumab is a monoclonal antibody that binds to the HER2 receptor, which is found on SKOV-3 cells. We demonstrate a noninvasive, impedance-based in vitro assay for the quantification of ADCC using the Maestro Z, and examine the response across multiple donors.
Materials and Methods
Cells and reagents
SKOV-3 (Cat. HTB-77) cells were obtained from ATCC (Manassas, VA). SKOV-3 media was composed of McCoy's 5A Medium Modified (ATCC, Cat. 30-2007) with 10% FBS (Gibco, Cat.16000044). Human PBNKs were isolated from healthy donors with human NK cell isolation kit (Miltenyi, Bergisch Gladbach, Germany, Cat. 130-092-657). PBNKs were stimulated for 24 hours using activation media composed of Glycostem basal growth media (GBGM, Glycostem, Kloosterstraat, The Netherlands), 10% FBS, 1000 U/ml IL-2 (Proleukin, VU Medisch Centrum), and 10 ng/ml IL-15 (CellGenix, Cat. 001413-050). CD56 and CD16 expression was measured by flow cytometry before starting the assay. Donor 1, 2, and 3 PBNKs were 93%, 86%, and 88% CD56+, respectively. Donor 1, 2, and 3 PBNKs were 95%, 87%, and 95% CD16+, respectively.
Antibodies used: anti-HER2 (anti-ErbB-2 (CD340)-APCVio770, clone 4D2, Miltenyi, Cat. 130-106-756), mouse IgG1-APC-Vio 770 (isotype control, clone IS5-21F5, Miltenyi, Cat. 130-113-759), trastuzumab (Bioconnect, Begonialaan, The Netherlands, Cat. HY-P9907), human IgG isotype control (Thermo Fisher, Waltham, MA, Cat. 02-7102), mouse anti-human IgG (H+L) AF488 (Thermo Fisher, Cat. A11013), anti-human CD16 (Biolegend, San Diego, CA, Cat. 302050).
Flow cytometry
To determine HER2 expression on SKOV-3 cells, 100,000 SKOV-3 cells/well were incubated with antiHER2 antibody (1:50) or isotype control (1:50) and 7- AAD (Beckman Coulter, Cat. A07704) to mark dead cells. Flow cytometry was performed to determine HER2 expression, and gates were drawn to exclude doublets and dead cells. Flow cytometry was also used to validate trastuzumab recognition of and binding to HER2. 100,000 SKOV-3 cells/well were incubated with 0.0001-10 µg/ml trastuzumab or isotype IgG control for 30 minutes at 4 °C, washed twice with FACS buffer, incubated with 0.1 µg/ml secondary antibody for 30 minutes at 4°C, and washed twice more with FACS buffer. Cells were then resuspended in 200 µL 7-AADcontaining FACS buffer (196 µL buffer+ 4 µL 7-AAD) and flow cytometry was run. Gating was performed to exclude doublets and dead cells and to determine trastuzumab binding capacity at all concentrations.
Maestro Z assay platform
The Maestro Z platform (Axion Biosystems) uses impedance measurements (ohms, O) to quantify the presence of cells on electrodes embedded in the bottom of the wells of CytoView-Z plates (Axion Biosystems). Cellular impedance is a well-established technique for measuring cell attachment, spreading, proliferation, coupling, membrane integrity (cell death), and subtle changes in cell conformation . Detection is noninvasive and label-free, so it can quantify dynamic cellular responses over minutes, hours, and days. The Maestro Z's built-in environmental chamber finely controls temperature and CO2, ensuring a consistent, optimal experimental environment.
Antibody cytotoxicity assay
To assess potential cytotoxic effects of trastuzumab, 50 µL of SKOV-3 media was added to each well of a CytoView-Z 96 plate, then the plate was docked on the Maestro Z platform for a media reference measurement. Integrated humidity reservoirs on the CytoView-Z 96 plate were filled with sterile water to maintain humidity. 40,000 SKOV-3 cells were added per well in 40 µLand allowed to adhere for 5 hours docked on the Maestro Z, which incubated the plate at 37°C and 5% CO2. Trastuzumab or isotype control were then added in 10 µL (for a total well volume of 100 µL) at concentrations ranging from 0.0001-10 or 0.01-10 µg/ml, respectively, and the plate was docked and measured for 24 hours.
PBNK-mediated ADCC assay
To measure PBNK cell-mediated killing, SKOV-3 cells were cultured as described above. After taking a media reference measurement (50 µL media/well), 40,000 SKOV-3 cells were seeded in 40 µL/well and docked on the Maestro Z for 6 hours at 37°C and 5% CO2.
After 5 hours, PBNK cells, which were isolated from healthy donors with human NK cell isolation kit (Miltenyi, Cat. 130-092-657) and stimulated for 24 hours with 1000U/ml IL-2,10 ng/ml IL-15, and 10% FBS in GBGM medium, were counted based on CD56+ cells and prepared as follows : for CD16 blocking, antiC016 antibody was added to PBNKs at 20 µg/ml at 37°C for 15 minutes. Subsequently, trastuzumab or isotype control were added to the SKOV-3 cells at a final concentration of 1 µg/ml in 10 µL/well.
After the 15-minute antibody incubations, PBNK effectors were added to the SKOV-3 cells at E:T ratios of 1:1, 1:3, and 1:10 in 100 µL/well, for a total well volume of 200 µL.
The plate was then returned to the Maestro Z and impedance was measured for 54 hours.
Calculation of cytolysis
%Cytolysis was used to quantify cell death and was calculated using the following equation:
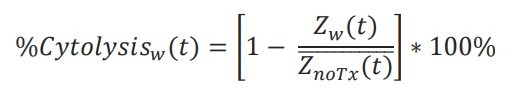
where 𝑍𝑛𝑜𝑇𝑥(𝑡) is a time series and the mean of the no treatment control wells.
%Cytolysis, kill time 50 (KT50), and area under the curve (AUC) of resistance or cytolysis were calculated and exported using AxIS Z software.
Results
Trastuzumab binds to HER2-expressing SKOV-3 cells without inducing cytotoxicity
For an antibody to bind to a cancer cell and facilitate ADCC, the target cell must express an antigen compatible with that specific antibody. In these experiments, the human ovarian cancer cell line SKOV-3 and the monoclonal antibody trastuzumab, which binds to HER2, were used. SKOV-3 cells were incubated with an anti-HER2 antibody, as well as an isotype control, and flow cytometry was used to determine the level of HER2 expression on the SKOV-3 cells. Greater than 90% of cells were determined to be HER2+ (Fig. 1A), confirming high expression.
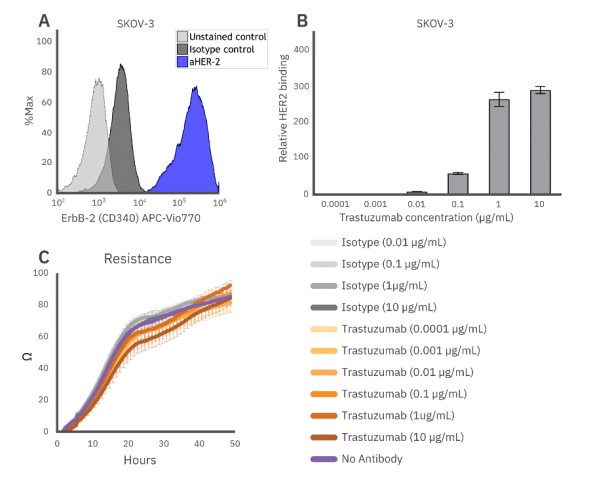
Figure 1: (A) HER2, the target of trastuzumab, is highly expressed in SKOV-3 cells. (B) Trastuzumab fully binds SKOV-3 at concentrations of 1 µg/ml. (C) Alone, trastuzumab does not induce cytotoxicity in SKOV-3 cells as indicated by no noticeable impact on SKOV-3 growth when compared to the no antibody or isotype control.
Trastuzumab was then added to SKOV-3 cells in increasing concentrations to assess the binding efficiency to HER2 on the cells via flow cytometry. Trastuzumab bound to the cells in a concentration-dependent manner, with binding saturation occurring at concentrations of 1 µg/ml or higher (Fig. 1B).
To accurately assess ADCC-specific killing, it is important to establish that the antibody does not induce cytotoxicity on its own. Potential cytotoxicity was measured via resistance on the Maestro Z. SKOV-3 cells were treated with trastuzumab and an isotype IgG control, then monitored on the Maestro Z for 48 hours. No cytotoxicity was observed at any concentration tested (ranging from 0.1 ng/ml - 10 µg/ml for trastuzumab and 10 ng/ml - 10 µg/ml for the isotype), evident by a lack of change compared to the no antibody control wells and continued tumor growth, over the entire 48-hour assay period for each group (Fig. 1C).
Antibody-dependent cellular cytotoxicity is observed with natural killer cells and trastuzumab
To measure the extent and potency of ADCC compared to standard natural killer (NK) cell-mediated cytolysis, NK cell-mediated cytolysis of SKOV-3 cells was assessed via impedance. An increase in resistance reflects an increase in tumor growth, while a decrease in resistance reflects immune-cell mediated killing. PBNK cells (Donor 1, 95% CD16+/93% CD56+) effectively killed SKOV-3 cells over 60 hours (Fig. 2A, teal). Area under the curve was used as a measurement of tumor growth over time (Fig. 2C), and % cytolysis (described in the methods) was tracked using non-treated SKOV-3 cells as a control (Fig. 2B).
To assess ADCC, 1 µg/ml trastuzumab or isotype IgG was added to SKOV-3 cells with the PBNK cells. Trastuzumab treatment enhanced NK-mediated cytotoxicity (Fig. 2A-D, orange), increasing cytolysis by 19% at 48 hours. Isotype control had no effect on NKmediated cytotoxicity (Fig. 2, light gray) at 48 hours. The ADCC effect observed with trastuzumab treatment was partially blocked by the addition of 20 µg/ml antiCD16 antibody (Fig. 2A-D, navy), suggesting a CD16- dependent mechanism for the enhanced cytolysis.
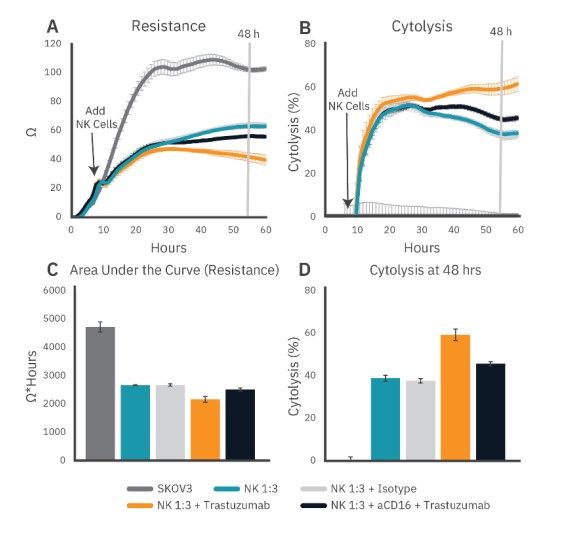
Figure 2: To measure real-time antibody-dependent cellular cytotoxicity, SKOV-3 cell attachment and growth was monitored for 6 hours using the Maestro Z platform. SKOV-3 cells were then incubated with trastuzumab or isotype control, while some PBNK cells were incubated with anti-CD16. After 15 minutes, the various PBNK conditions were added to SKOV-3 cells at a 1:3 E:T ratio and (A) resistance was measured for an additional 54 hours. (B) Cytolysis was plotted using the no treatment control as described in the methods. (C) Area under the curve (resistance) was calculated to demonstrate the full time course response. (D) The difference in cytolysis between PBNK cells alone and with trastuzumab demonstrates ADCC, which is partially blocked by anti-CD 16.
ADCC is effector:target ratio-dependent
For ADCC, as for other immunotherapy potency assays, effector:target (E:T) ratio must be carefully considered. At high E:T ratios, killing may be too rapid and/or complete to detect an ADCC-specific effect, whereas low E:T ratios may not provide enough cytolysis.
Here, trastuzumab (1 µg/ml)-mediated ADCC was evaluated using E:T ratios of 1:1, 1:3, and 1:10, as described above. PBNK cells from Donor 1 were used (95% CD16+/93% CD56+). While ADCC was observed at all ratios, the effect was strongest at the lower ratios (Fig. 3). Area under the curve (resistance) and cytolysis at 48 hours post-addition show a smaller response at the 1:1 E:T ratio as PBNK cells alone reached near total cytolysis. The increased cytolysis observed between PBNK cells alone and with trastuzumab at lower E:T ratios demonstrates the influence of E:T ratios on ADCC detection.
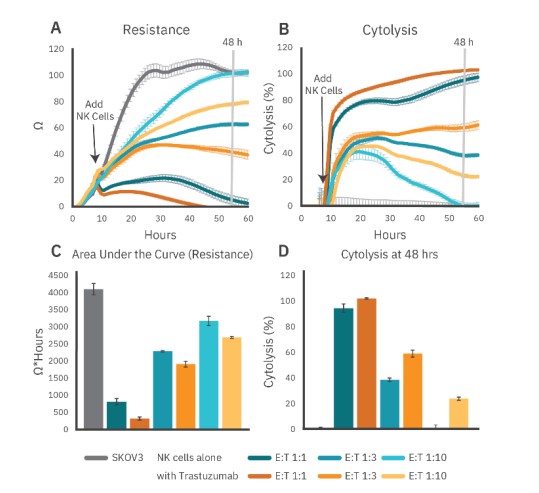
Figure 3: PBNK cell-mediated cytolysis at different E:T ratios in the presence or absence of trastuzumab (1 µg/ml) as shown by (A) resistance or (B) cytolysis. (C) Area under the curves (resistance) and (D) cytolysis at 48 hours show a smaller ADCC response at higher E:T ratios as the PBNK cells alone reached near total cytolysis.
ADCC is obvserved across donors
Donor variability is another important consideration for both assay development and efficacy of potential immunotherapies. Conclusion A 120 100 l so V> ~ 60 2 (S 40 20 C 120 100 l so "' ~ 60 0 & 40 20 To evaluate donor variability, we assessed cytolysis and ADCC capability of NK cells from several donors using the assay described above. NK-mediated cell killing and ADCC via trastuzumab were observed across all donors, particularly at the 1:3 E:T ratio, as evidenced by the % cytolysis (Fig. 4). Less ADCC was observed at 1:1 and 1:10 ratios for donors 2 and 3. These results support not only the efficacy of trastuzumab to facilitate ADCC, but also the reliability of the Maestro Z impedance assay to detect cytolysis and quantify the magnitude and kinetics of ADCC.
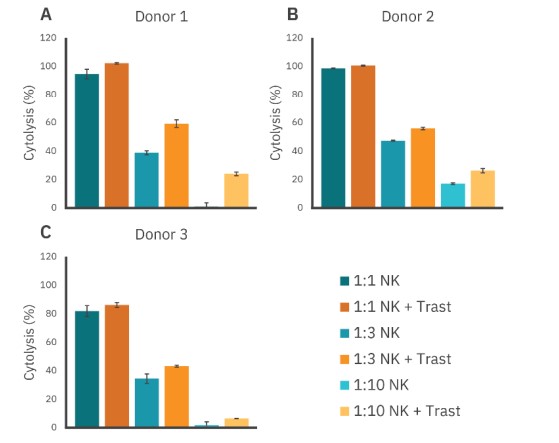
Figure 4: Antibody-dependent cellular toxicity across donors. Cytotoxicity from three donors were tested with and without trastuzumab. At the 1:3 E:T ratio, trastuzumab enhances immune cell-mediated killing.
Conclusion
The Maestro Z allows for simple, noninvasive, real-time monitoring of immune cell-mediated killing of target cancer cells, providing a sensitive, quantitative assay for evaluating immune cell potency in vitro. Continuous monitoring of PBNK-mediated cell killing provides a detailed look at the influence of anti-tumor antibodies like trastuzumab. ADCC-specific killing was demonstrated against cancer cells and quantified across donors. The ADCC observed could be blocked by a CD16-dependent mechanism and was sensitive to E:T ratios.
By applying the principles demonstrated in this assay, immune cell-enhancing therapeutics can be evaluated in vitro.
About Glycostem Therapeutics
Glycostem Therapeutics is focused on the development of stem cell-derived Natural Killer cells (NK cells) as a medicinal asset in the fight against cancer. NK cells are the new star in the domain of cellular immunotherapy, due to their tightly regulated "natural killing" of cancer cells; they play an important role in control and even cure of both solid and hematological malignancies, like Acute Myeloid Leukemia (AML) and Multiple Myeloma (MM).
References
- Mando et al. "Targeting ADCC: A different approach to HER2 breast cancer in the immunotherapy era." The Breast. (2021).
- Lo Nigro et al. "NK-mediated antibody-dependent cell-mediated cytotoxicity in solid tumors: biological evidence and clinical perspectives." Annals of translational medicine. (2019).
- David Zahavi et al. "Enhancing antibody-dependent cell-mediated cytotoxicity: a strategy for improving antibody-based immunotherapy" Antibody Therapeutics. (2018).
- Tóth Get al. "Quantitating ADCC against adherent cells: Impedance-based detection is superior to release, membrane permeability, or caspase activation assays in resolving antibody dose response." Cytometry A. (2017)